Table 1. Optimization of reaction parameters.
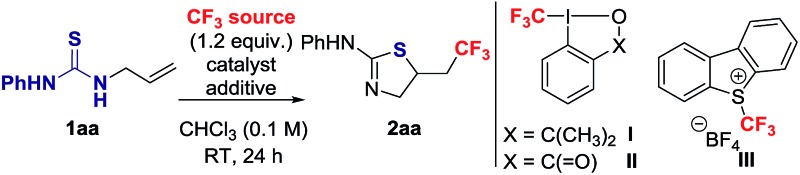
| ||||
| Entry | CF3 source | Catalyst | Additive | Yield a (%) |
| 1 | I | — | — | 7 |
| 2 | II | — | — | 33, 21 b |
| 3 | III | — | — | 12, 7 b |
| 4 | II | — | TFA (2 equiv.) | 76, 62 c |
| 5 | II | — | TFA (1 equiv.) | 69 |
| 6 | III | — | TFA (2 equiv.) | 13 |
| 7 d | II | — | TFA (2 equiv.) | 59 |
| 8 | II | A (5 mol%) | — | 21 |
| 9 | II | A (100 mol%) | B (1 equiv.) | 33 |
| 10 e | II | C (5 mol%) | — | 20 |
| 11 e | II | D (2 mol%) | — | 31 |
| 12 e | III | C (5 mol%) | — | 40 |
| 13 e | III | D (2 mol%) | — | 38 |
a Determined by 19F NMR integration relative to an internal standard (C6H5CF3).
b Reaction at 60 °C.
c Reaction time is 1 h.
d Reaction in CH3CN.
e 14 W bulb as light source (λ max = 452 nm). A = Cu(CH3CN)4PF6. B = 1,10-phenantroline. C = Ru(bpy)3(PF6)2. D = methylene blue. TFA = trifluoroacetic acid.
