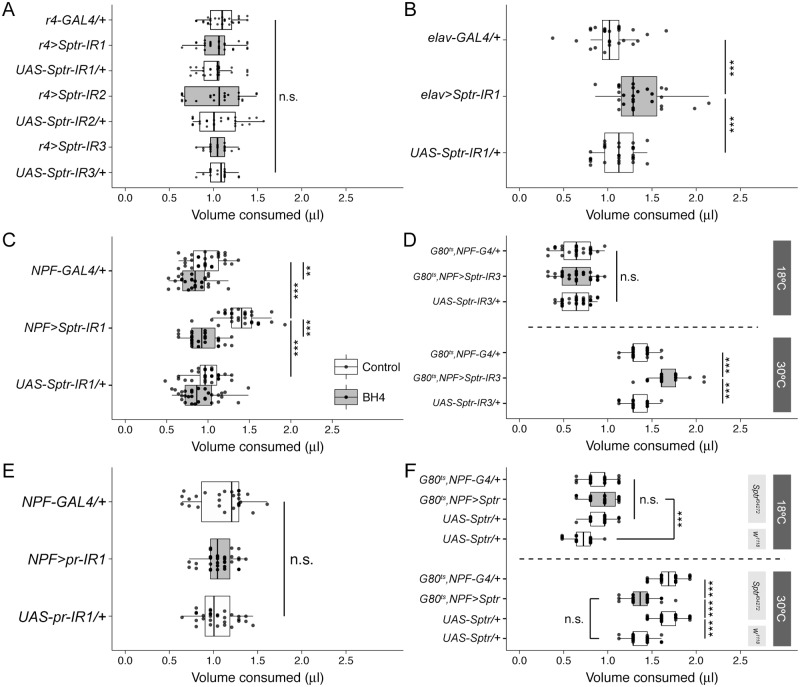
Fig 4. Proper appetite regulation depends on Sepiapterin Reductase (Sptr) expression in neuropeptide F (NPF) neurons, not the fat body.
(A) Fat body-specific knock-down of Sptr using three different RNA intereference (RNAi) lines (gray boxplots) does not alter feeding compared to heterozygous controls (white boxplots; n = 20–29). See also S6A Fig. (B) Pan-neuronal knock-down of Sptr increases feeding (n = 27–28). See also S6C and S7 Figs. (C) Knock-down of Sptr in NPF neurons increases feeding, and this hyperphagia can be rescued by pre-feeding the flies 0.17 mg/mL tetrahydrobiopterin (BH4; n = 30). See also S8A and S8B Fig. (D) Limiting the NPF neuron-specific Sptr knock-down (gray boxplots) to the adult stage using a temperature-sensitive tub-GAL80ts still increases feeding beyond that of heterozygous controls (white boxplots; n = 30). Importantly, there is no change in feeding at 18°C. (E) Knock-down of pr in NPF neurons has no effect on feeding amount (n = 27–34). (F) Adult stage NPF neuron-specific Sptr overexpression (gray boxplots) rescues the hyperphagia of Sptrf04272 mutant flies to the level of a w1118 background control (n = 30).