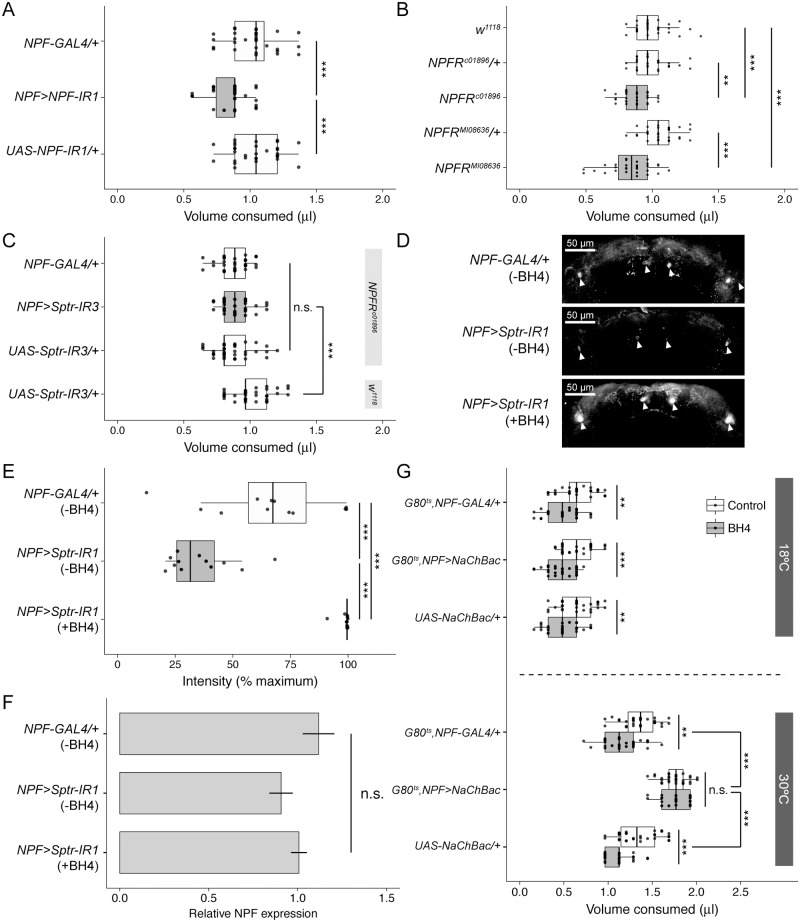
Fig 5. Tetrahydrobiopterin (BH4) acts via neuropeptide F (NPF)-NPFR signaling to modulate appetite.
(A) NPF knock-down in NPF neurons (gray boxplot) reduces ad libitum feeding (n = 30) below that of heterozygous controls (white boxplots). (B) Two hypomorphic NPF receptor mutants, NPFRc01896 and NPFRMI08636 (gray boxplots), eat less than w1118 and their heterozygous controls (white boxplots; n = 30). (C) In the NPFRc01896 mutant background, knock-down of Sepiapterin reductase (Sptr) in NPF neurons fails to enhance feeding (n = 35). (D) Staining of dissected brains with an NPF-specific antibody. NPF neuron-specific knock-down of Sptr reduces NPF accumulation in NPF neuron cell bodies. Pre-feeding with 0.34 mg/mL BH4 dramatically increases NPF accumulation. Arrowheads indicate the NPF neuron cell bodies. (E) Boxplots comparing the NPF signal intensities of 12 stained cell bodies from three brains for each genotype. All brains were imaged with the same confocal settings. (F) Relative NPF gene expression normalized to three control genes under the same conditions as (D) and (E) as measured by quantitative polymerase chain reaction (qPCR). Each bar indicates the mean ± standard error of the mean (s.e.m.) of three technical replicates of each of two biological samples (RNA extracted from 40 adult brains) amplified using two different NPF-specific primer sets. (G) Continuous hyper-activation of adult NPF neurons using the bacterial sodium channel NaChBac induces hyperphagia. This hyperphagia is not rescued by pre-feeding with 0.34 mg/mL BH4 (n = 30). Underlying numerical data for this figure can be found here: http://dx.doi.org/10.5061/dryad.8hm82.