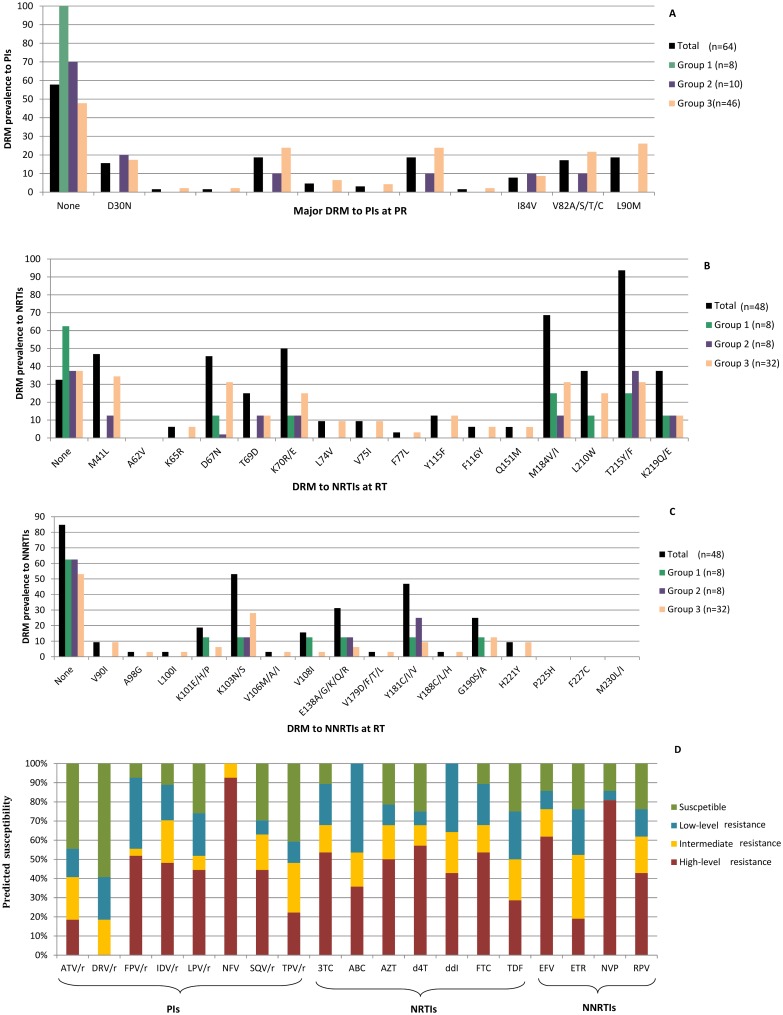
Fig 1. DRM in 64 LPV/r experienced paediatric patients and predicted drug-susceptibility in those carrying resistant viruses.
Fig 1 legend: (A) DRM at PR associated with PI resistance in 64 HIV-1-infected paediatric patients with available PR resistant data (PR sequence or resistance profile to PI) during LPV/r exposure. (B) and (C) DRM at RT associated with NRTI or NNRTI resistance, respectively, in 46 HIV-1-infected paediatric patients with available RT sequence or resistance data to RT inhibitors during LPV/r experience. (D) Predicted susceptibility to antiretrovirals in viruses carrying DRM to IP (n = 27), to NRTI (n = 28) or to NNRTI (n = 21) according to Standford Algorithm. NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-NRTI; r, ritonavir used for boosting; ATV/r, boosted-atazanavir; DRV/r, boosted-darunavir; FPV/r, boosted-fosamprenavir; IDV/r, boosted-indinavir; LPV/r, boosted-lopinavir; NFV, nelfinavir; SQV/r, boosted-saquinavir; TPV/r, boosted-tipranavir; 3TC, lamivudine; ABC, abcavir; AZT, zidovudine; d4T, estavudine; ddI, didanosine; FTC, emtricitabine; TDF, tenofovir; EFV, efavirenz; ETR, etravirine; NVP, nevirapine; RPV, rilpivirine.