Figure 1. Expression of oncogenic KRASG12D in required for the onset and maintenance of primary and metastatic pancreatic ductal adenocarcinoma (PDAC).
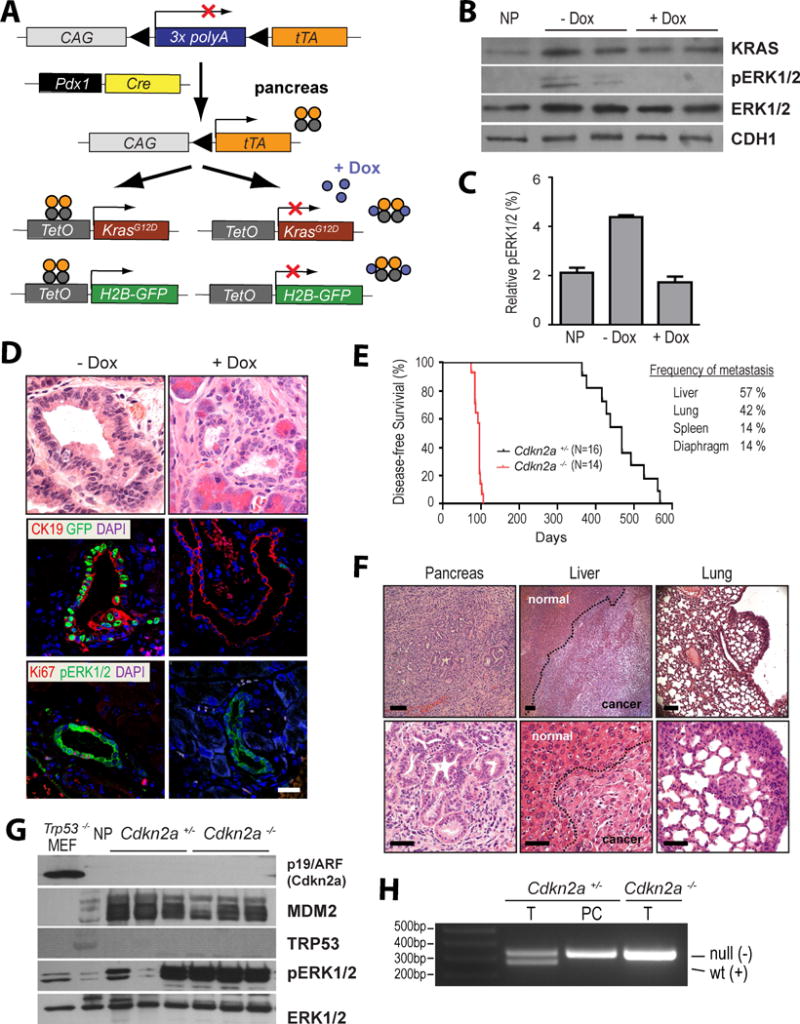
A. Generation of a genetically engineered mouse model that permits a temporally and spatially controlled expression of oncogenic KRAS and a H2B-GFP reporter in the pancreas in a doxycycline (Dox)-repressible manner (TET-OFF). B. Total KRAS protein expression as well as downstream activation of ERK1/2 in triple transgenic mice before and after administration of Dox; NP, normal pancreas of a littermate control that lacks the TetO-KRASG12D transgene. C. Quantitative analysis of relative ERK1/2 activation as determined by capillary electrophoresis of triplicate sets of tissues shown in panel B on a ProteinSimple NanoPro 1000 machine. D. H&E stained histological sections and immunofluorescent labeling of CK19, GFP, Ki67, and pERK1/2 in pancreatic specimens of 3-month-old Pdx1-Cre, CAG-LSL-tTA, TetO-KRASG12D, TetO-H2B-GFP quadruple transgenic mice prior to (-Dox) and after 7 days of Dox treatment; bar represents 50 μm. E. Kaplan–Meier survival plot of mice that conditionally express mutant KRAS in a Cdkn2a heterozygous (Cdkn2a+/−) or homozygous knockout background (Cdkn2a−/−). The table illustrates the relative incidence of metastatic lesions in these mice. F. Representative images of H&E stained sections of primary PDAC as well as metastatic lesions in liver and lung. G. Expression of p19/Arf, MDM2, and p53 as well as activation of ERK1/2 in primary pancreatic tumors of mice that express oncogenic KRAS in the Cdkn2a heterozygous and homozygous knockout background; NP, normal pancreas of a wildtype mouse. Trp53-deficient mouse embryonic fibroblasts (MEF) served as additional control. H. PCR assay to validate the loss of exon 2 of Cdkn2a in the DNA of purified pancreatic cancer cells (PC) of a mouse that was genotyped as a heterozygous Cdkn2a knockout (Cdkn2a+/−) using DNA from the tail (T). Tail DNA from a homozygous Cdkn2a knockout (Cdkn2a−/−) was used as a control.
