Abstract
Introduction
The use of propofol in endoscopy is becoming more prevalent both in Europe and North America. Potential advantages over conscious sedation include controlled deep sedation for therapeutic endoscopy and improved patient satisfaction. A new anaesthetist-led propofol-based day-case sedation service was introduced within the endoscopy unit at the Royal Liverpool University Hospital in April 2011.
Aims
To evaluate this new service of anaesthetist-led propofol-based sedation for safety, compliance with current guidelines and satisfaction (patient, anaesthetist and endoscopist).
Design
A prospective, service evaluation audit of a new, weekly, anaesthetist-led propofol-based sedation service. Administrative records, anaesthetic notes and satisfaction scores (1=very dissatisfied; 5=very satisfied; patients, anaesthetists, endoscopists) and the ‘patient journey’ were evaluated for 40 consecutive patients treated over 18 weeks. Outcomes were measured against current British Society of Gastroenterology/Royal College of Anaesthetists guidelines.
Results
All procedures were completed (100% intention-to-treat rate), all patients were discharged on the day of the procedure and none were readmitted within 7 days. Adverse events were minor (10%) and there were no deaths within 30 days. The median satisfaction score was 5 for patients, anaesthetists and endoscopists. The additional cost for provision of such a service included the services of the anaesthetist (one programmed activity) and operating department personnel and for drugs (propofol). The demand for the service rapidly increased.
Conclusions
Anaesthetist-led propofol-assisted endoscopy is safe in a day-case endoscopy unit and is associated with high satisfaction scores for patients, anaesthetists and endoscopists. There is a high demand for this service in this UK endoscopy day-case unit.
Keywords: Endoscopy
Introduction
Endoscopic procedures in UK hospitals are mostly performed in day-case endoscopy units under conscious sedation. Studies1–2 and a meta-analysis3 have established that conscious sedation provides a high level of patient and physician satisfaction with low adverse effects and associated higher procedural completion rates.
The standard sedation practice in the UK is that diagnostic upper gastrointestinal endoscopy is usually performed either on unsedated patients with a short-acting pharyngeal anaesthetic (xylocaine; 10 mg per spray) alone or under conscious sedation in combination with a short-acting intravenous benzodiazepine (midazolam). However, more complex procedures such as endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasound (EUS) and therapeutic procedures are performed under conscious sedation using a combination of intravenous benzodiazepine (midazolam) and an opioid analgesic such as pethidine or fentanyl. Lower gastrointestinal endoscopic procedures are performed either on unsedated patients or under conscious sedation with a combination of intravenous benzodiazepine and opioid analgesics. However, some patients do not tolerate endoscopic procedures even under maximum doses of conscious sedation and therefore subsequently undergo endoscopy under general anaesthesia in a surgical operating theatre.
A newer technique of deep (unconscious) sedation with propofol may for many represent an alternative to general anaesthesia that may be potentially provided within a day-case endoscopy unit. Propofol is an ultra-short-acting agent that provides sedative, hypnotic and amnesic effects but has no analgesic properties.4 The time of onset of action is 30–60 s following intravenous administration and the duration of effect lasts between 4 and 8 min.5 It is widely used to induce general anaesthesia, and may do this for some patients at dosage levels that would provide insufficient sedation for others: individual and careful titration, even when using target-controlled infusion (TCI) devices, is essential. Deep sedation with propofol may also be associated with significant hypotension and respiratory depression. Furthermore, while reversal agents are available for benzodiazepines (flumazenil) and opioids (naloxone) in the event of oversedation causing respiratory depression, hypotension, stupor, coma or apnoea, none exists for propofol. Therefore, facilities for immediate airway, positive pressure ventilation and cardiovascular support are essential when using propofol for sedation.
Several randomised controlled studies have addressed the safety and efficacy of propofol sedation for endoscopy (as monotherapy and in combination with traditional sedatives).6–10 Meta-analysis on the use of propofol for endoscopic procedures has demonstrated the lower odds of cardiopulmonary complications associated with propofol use compared to traditional sedatives.11 Moreover, propofol use was found to be associated with faster recovery time even after prolonged administration, earlier food intake, early discharge from endoscopy units and quicker return to normal activity.11 It was also shown to be associated with higher satisfaction scores from patients and endoscopists.11
There has been an increasing trend to use propofol sedation for endoscopic procedures in Europe and North America. In a nationwide questionnaire study in Germany, 74% of cases were performed under propofol.12 In Italy, although benzodiazepines and opioids were still being used for conscious sedation for the majority of patients requiring gastroscopy or colonoscopy, approximately 50% of ERCP/EUS procedures were performed under propofol sedation.13 In Greece, 34% of all endoscopies were performed under propofol.14 In a single centre in Romania, 97% of endoscopic procedures were reported to be performed under propofol sedation administered by an anaesthetist.15 In the USA, 25% of all gastrointestinal endoscopic procedures were performed under propofol sedation.16
The British Society of Gastroenterology (BSG) has published guidelines for the safe use of sedatives in endoscopic procedures and more recently has published guidelines jointly with the Royal College of Anaesthetists (RCoA) for the use of propofol in ERCP and other complex therapeutic upper gastrointestinal endoscopy.17 This document defines the equipment and environmental requirements as well as the manpower and training needs that are essential to establish and deliver such a service.
At the Royal Liverpool University Hospital, all endoscopic procedures including ERCP and EUS were being performed under conscious sedation in a day-case endoscopy unit. Patients intolerant of the procedure under conscious sedation had the procedure subsequently performed under general anaesthesia in the main operating theatre suite. The endoscopy unit performs over 15 000 endoscopic procedures annually, and is a regional tertiary referral centre for complex therapeutic procedures. With an increasing number of referrals there is an increasing demand for procedures to be performed under general anaesthesia. An anaesthetist-led propofol sedation service (ALPS) was therefore introduced to address this demand.
The aim of this study was to evaluate this service for compliance with national guidelines, for safety (adverse events, 7-day readmission and 30-day death rates), for provider, user and consumer satisfaction levels, and to identify areas for further improvement and development.
Methods
Service evaluation audit approval
The audit was approved and registered by the Royal Liverpool University Hospital Trust's clinical effectiveness and audit committee as a joint directorate audit between gastroenterology and anaesthetics.
Setting and patient flow
All endoscopic procedures were performed at the Royal Liverpool and Broadgreen University Hospitals NHS Trust (RLBUHT) at the Royal Liverpool Hospital site. Procedures were either performed in a procedure room in the endoscopy unit or in a dedicated fluoroscopy room in the radiology department located adjacent to the endoscopy unit. ALPS was provided weekly on a dedicated deep sedation list (one session of 3.5 h). A maximum of three patients was scheduled per list. If procedures involved fluoroscopy, this was coordinated with the radiology department so that the room was booked well in advance and staffed appropriately by a radiographer for the duration of the procedure. Procedures were clustered together so that those requiring fluoroscopy were performed at the same session/list. The administrative staff of the endoscopy unit coordinated listing of patients. Patients were contacted by telephone and subsequently information was sent by post. A consultant endoscopist vetted all requests for propofol sedation. Only those patients who had failed previous conscious sedation or were undergoing complex therapeutic procedures such as HALO and ampullectomy were selected. All endoscopic procedures were performed by a consultant endoscopist, assisted by trained endoscopy staff, and ALPS was provided by a consultant anaesthetist assisted by an operating department practitioner. A dedicated ‘deep-sedation’ drug trolley was introduced. Stocks were maintained through regular inventory by the anaesthetic team. Additional equipment including that for capnography was borrowed from the anaesthetic department on a sessional basis.
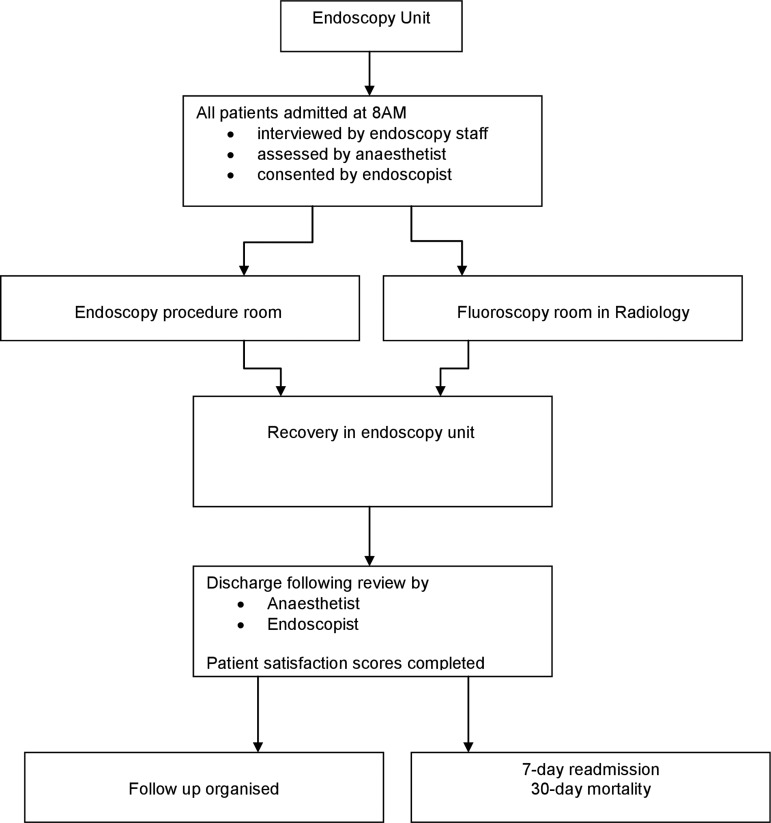
Upon arrival at the day-case endoscopy unit patients were interviewed by the endoscopy staff and then assessed by the consultant anaesthetist. Following the completion of the procedure, all patients were recovered in the ‘recovery bay’ of the day-case endoscopy unit. Before discharge, patients were reviewed by both the anaesthetist and endoscopist and all patients were issued post-procedure care leaflets. This patient flow is illustrated in figure 1.
Figure 1.
Patient Flows: This described the patient flows for the propofol lists in the 2 main remote areas of endoscopy room and XR.
Study design
This was a prospective service evaluation audit performed from June to December 2011 on all patients listed for the weekly ALPS at the RLBUHT. Using a standardised audit proforma administrative, anaesthetic, clinical, endoscopic and satisfaction data were collected. Information including indications for endoscopy and reasons for deep sedation was obtained. All data were anonymised in accordance with the Data Protection Act, and the data were entered onto an excel spreadsheet (Microsoft), which was held on a secure hospital server. The data were collected and analysed by a post-CCT gastroenterology fellow who had no association with the list during the study period.
Satisfaction scoring
A five-point satisfaction scale (scoring system: 1=very dissatisfied; 2=somewhat dissatisfied; 3=neither satisfied nor dissatisfied; 4=somewhat satisfied; 5=very satisfied) was used for evaluating patient, endoscopist and anaesthetist satisfaction with the propofol sedation. Satisfaction scores for both endoscopist and anaesthetist were completed at the end of each procedure, and for patients before their discharge from the day-case endoscopy unit.
Outcome measures
Our primary aim was to evaluate the service against current RCoA/BSG guidance, and secondary aims included a ‘snapshot’ assessment of the efficacy, safety and provider/user/consumer satisfaction with ALPS. Standards have been published jointly by RCoA and BSG regarding the provision of deep sedation with propofol in remote areas. The RCoA defines a remote site as ‘any location at which an anaesthetist is required to provide general/ regional anaesthesia, or sedation away from the main theatre suite and/or anaesthetic department and in which it cannot be guaranteed that the help of another anaesthetist will be available. This may be either within or away from the base hospital.’18 The guidance document from the BSG and RCoA includes standards with regard to:
Personnel responsible for administering propofol for sedation and training;
Patient selection;
Minimum requirements for equipment and the environment; and finally
Minimum staffing levels and generic training.
The audit standards therefore included (table 1):
All deep sedation provided by a consultant anaesthetist;
Patient assessed by the anaesthetist before provision of deep sedation;
Equipment and environment;
A self-contained endoscopy unit including an adequately staffed recovery area;
Piped oxygen and suction in all areas;
Appropriate equipment for supporting respiration;
Appropriate ‘tilting’ trolleys;
- Monitoring with
- pulse oximetry
- electrocardiogram
- automatic non-invasive blood pressure monitoring
- continuous waveform capnography;
Full resuscitation facilities;
Dedicated trained staff to assist both anaesthetist and endoscopist separately.
Results
Patient administration and demographics
Median waiting time for the ALPS was 8 weeks (IQR 3–10) compared to a median of less than 6 weeks for other endoscopic procedures for the unit. Of the 50 patients listed, 40 (80%) patients had the procedure performed. In the 10 that did not have a procedure, four (8%) patients did not attend for the procedure and six (12%) patients had their procedure cancelled on the day of the procedure following review by the anaesthetist. Cancellations on the day of the procedure were due to the presence of significant comorbidity, including obesity (n=4), anticipated difficult airway (n=1) or intercurrent chest infection (n=1) precluding safe sedation with propofol. Those patients cancelled on the day of procedure were subsequently referred for the procedure to be performed under general anaesthesia. Of the 40 patients, 19 (47.5%) were women with a median age of 53 years (range 18–80 years) and median American Society of Anesthesiologists grade of 2 (range 1–3).
Indication for propofol
The procedures performed under deep sedation and their indications are detailed in table 1. Twenty-four (60%) patients had previous unsatisfactory endoscopy under conscious sedation due to underlying phobia, extreme anxiety, intolerable pain (with colonoscopy) and previous stricture dilation. The remaining 16 (40%) were undergoing radio frequency ablation of dysplastic lesions in Barrett's oesophagus and were therefore advised to have the procedure performed under deep sedation (table 1).
Table 1.
Total number of patients, endoscopic procedures, indications and reasons for deep sedation with propofol. The majority of patients were those that had failed under conscious sedation or were therapeutic endoscopy of RFA
| Endoscopic procedure | Total Number of patients (%) | Reason for propofol sedation | Indication for procedure |
|---|---|---|---|
| OGD | 2(5%) | Failed oral intubation (n=2) | Diagnostic |
| Colonoscopy | 14 (35%) | Pain (n=8) | Diagnostic |
| Phobia (n=2) | Diagnostic | ||
| Stricture (n=2) | Diagnostic | ||
| Not tolerated (n=2) | Diagnostic | ||
| EUS | 2 (5%) | Failed oral intubation (n=2) | Diagnostic |
| ERCP | 3 (7.5%) | Failed conscious sedation (n=3) | Therapeutic |
| Ampullectomy | 2 (5%) | Nature of procedure (n=2) | Therapeutic |
| Radiofrequency ablation (RFA) of dysplastic oesophageal lesion (HALO®) | 16 (40%) | Nature of procedure (n=16) | High grade dysplasia for endoscopic management |
| Small bowel enteroscopy | 1 (2.5%) | Nature of procedure (n=1) | Diagnostic |
Table 2.
Compliance and modifications required to achieve compliance with national standards. The majority was achieved with the exception of table tilting and piped suction in the room
| RCoA/BSG standards criteria | Compliance | Modification required to meet 100% compliance |
|---|---|---|
| ALPS provided by Consultant Anaesthetist | 100% | N/A |
| Patient assessed by Anaesthetist | 100% | N/A |
| Self-contained endoscopy unit and recovery area | 100% | No |
| Piped oxygen and suction in all areas | 65% | Yes |
| Appropriate equipment for supporting respiration | 98% | Yes |
| Appropriate tilting trolleys | 50% | no |
| Monitoring with continuous waveform capnography | 93% | Yes |
| Full resuscitation facilities | 100% | no |
| Other monitoring devices:1. Pulse oximetry2. ECG3. NIBP | 1. 100% 2. 98% 3. 100% |
1. No 2. Yes 3. No |
| Staffing: trained assistant to Consultant anaesthetist | 100% | N/A |
| Staffing: trained assistant to Endoscopist | 100% | N/A |
| 1:1 nursing at recovery | 75% | YES |
N/A: Not applicable
Procedural duration, completion rates and day-case endoscopy unit discharges
The intention-to-treat completion rate was 100% and the mean duration per endoscopy was 33 min (range 10–70 min). All patients were discharged on the same day.
Medications administered
Overall, the median dose of propofol administered was 355 mg (IQR 171–463; mean dose 364.8 mg), fentanyl was 75 μg (IQR 43–100; mean dose 62.5 μg) and the median dose of midazolam was 0 (IQR 0–3; mean dose 1.25 mg). In addition all patients received 1 g of paracetamol and 4 mg of intravenous ondansetron (anti-emetic). No reversal agents were required for any of the procedures.
Compliance with guidelines
The compliance with current RCoA/BSG standards is detailed in table 2 along with the modifications required to achieve 100% compliance with current standards. All patients were assessed and deep sedation was provided by a consultant anaesthetist. Although most standards were met, in certain areas, such as provision of capnography (93% standards achieved), use of appropriate tilting trolley (50% standards achieved) this was not possible; in some areas 100% compliance was achieved only through modification of existing equipment (table 2).
Satisfaction scores
Acceptability for the ALPS was good overall, with very high satisfaction scores (median score 5) not only for patients but also endoscopists and anaesthetists, with a maximum score of 5 and minimum of 3 (figure 2).
Figure 2.
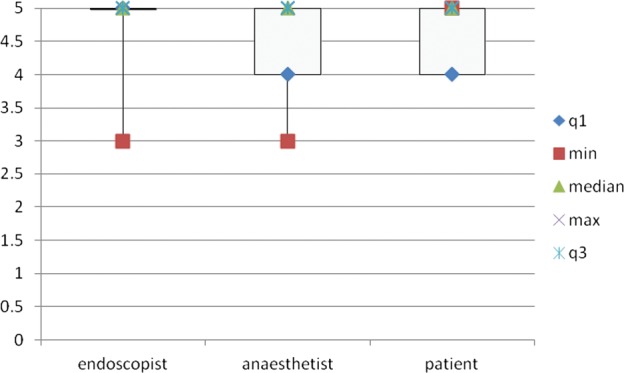
Satisfaction Scores: This shows the box plots (median with IQR) of the patient, endoscopist and anesthetist satisfaction scores which attained maximum of 5 (median) for all three groups.
Complications and adverse events
All adverse events related to deep sedation were minor by ASGE criteria,18 occurring in four (10%) patients. This included transient hypoxia (defined as SpO2 < 90%) in two (5%) patients. This was treated with simple jaw thrust in one patient while the other patient required temporary insertion of an oropharyngeal airway device. Mild transient hypotension (defined as blood pressure < 90/60 mm Hg) was observed in one (2.5%) patient and persistent hypotension requiring vasopressor use was seen in another patient (2.5%). Procedural complications were minor—with one patient developing minor bleeding. Once haemostasis was achieved, the procedure was satisfactorily completed and the patient was discharged on the same day. All patients (100%) were therefore discharged from the day-case endoscopy unit on the same day with no emergency admissions required. This is illustrated in figure 3. There were no 7-day readmissions or 30-day mortality.
Figure 3.
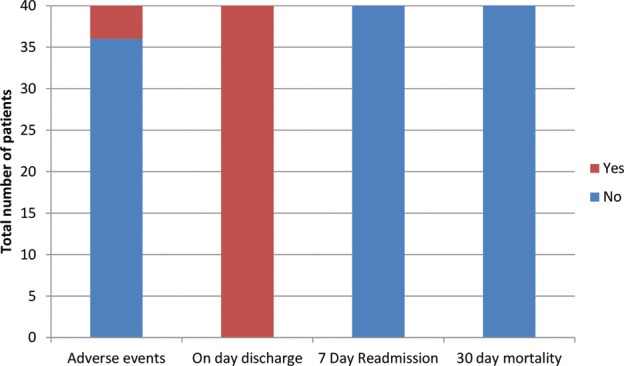
Adverse events, 7 day readmission and 30-day mortality; There was a 10% rate of minor adverse events, with all patients discharged as a day-case, and no readmission or 30 day mortality.
Discussion
Traditionally, gastrointestinal endoscopic procedures in the UK are performed on patients who are unsedated or under conscious sedation with intravenous benzodiazepine (midazolam) either as a sole agent or in combination with an intravenous opioid (fentanyl or pethidine). The maximum dose of these medications that can be administered by endoscopists is now monitored and regulated. Patients who do not tolerate the procedure under conscious sedation are subsequently referred for the procedure to be performed under general anaesthesia in an operating theatre environment. This exposes them to the risks of general anaesthesia and in addition frequently delays diagnostic and treatment pathways. Our experience has shown that deep sedation with propofol is a safe and effective alternative to general anaesthesia in a UK endoscopy day-case unit.
Several studies have addressed the safety and efficacy of non-anaesthesiologist administered propofol sedation for endoscopic procedures.9 10 However, in those studies propofol was administered by trained personnel (gastroenterologist/nurse) who was not the endoscopist. The European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates and the European Society of Anaesthesiology have issued guidelines for non-anaesthesiologist administration of propofol for gastrointestinal endoscopy.19 Similar guidelines were issued by the American Society of Gastrointestinal Endoscopy following legislation in the USA regarding the use of propofol by non-anaesthesiologists.20 UK national guidance from the BSG and the RCoA recommend that propofol must be administered only by an appropriately trained anaesthetist or by a physicians’ assistant supervised by a consultant anaesthetist at all times. Until such time that a training programme is established for non-anaesthesiologist administered propofol sedation, this will continue to remain an anaesthetist-led service within the UK.
Within the literature, it remains ambiguous whether such a service can be provided safely and efficiently in a ‘non-operating theatre room’ setting that is in a remote site. However, our data have shown that it is possible to run a safe effective remote site service on a day-case basis with very minimal adverse events with 100% day-case endoscopy unit discharges and no readmission or mortality. The current literature suggests that the provision of deep sedation in remote sites presents a number of challenges and risks, including isolated environment, ability to deal safely with sedation and procedural-related risks, availability of all required equipment, availability of a high level of assistance and difficulties with communication.21 Consequently, the RCoA and BSG have produced guidelines,17 with recommendations for deep sedation in remote sites. These include that this should be provided by appropriate experienced consultants and that equipment, including anaesthetic equipment and other equipment essential for resuscitation and life support, should be standardised across all areas providing such services. In keeping with current guidelines, all of our deep sedation lists were delivered by a consultant anaesthetist supported at all times by a fully trained and dedicated anaesthetic assistant. This allowed the provision of an identical level of anaesthetic support to that provided within the main operating theatre suite. These lists were also identified as a training resource and actively used for the teaching of deep sedation and remote site working techniques to trainee anaesthetists. Resuscitation equipment was immediately available in both radiology and endoscopy departments. In addition, a deep sedation trolley was developed and maintained by the anaesthetists so that all essential equipment was readily available. Regular inventory by the anaesthetists ensured that stocks were maintained and any additional equipment procurement was made through the endoscopy unit. In addition, the guidelines state that mandatory monitoring at remote sites should be as for any location where anaesthesia is conducted: a pulse oxymeter, non-invasive blood pressure cuffs, electrocardiogram and end-tidal carbon dioxide are a minimum requirement.20 These were all made available for all of our deep sedation lists. Before the start of each procedure a team-based safety briefing as well as individual patient safety checks, including the WHO checklist, were performed. Post-procedural patient care was undertaken in the recovery area of the endoscopy unit with regular monitoring by a dedicated nurse. Through procurement of dedicated capnography equipment, tilting trolleys and other equipment we hope to achieve 100% compliance with current guidelines. The trust (RLBUHT) has just received approval for the building of a new hospital, and measures are already in place in the design of the new endoscopy unit to accommodate the necessary equipment required for the provision of this service.
The indications for the list were 60% related to intolerance of endoscopy under conscious sedation and 40% for complex therapeutic procedures, which was predominantly HALO. As for the latter, HALO is a complex, therapeutic, time-consuming, procedure associated with multiple intubations and significant patient discomfort. Our previous experience with conscious sedation for this procedure was that the regulated maximum dose limit for midazolam was inadequate. Consequently, this deep sedation list has been invaluable for the treatment of these patients. With our unit being a tertiary referral centre for complex therapeutic endoscopic procedures the facility to use propofol had been much awaited. With our increase in capacity we are now able to offer all HALO, all upper gastrointestinal endoscopic mucosal resections and a selection of hepatobiliary interventions (ampullectomy) within this service. Overall, in the UK, we have been particularly slow in adopting this practice: within much of Europe and North America it is standard to use propofol for therapeutic endoscopy. The 100% completion of procedures in patients for whom traditional conscious sedation had proved inadequate well illustrates the effectiveness of deep sedation with propofol within a day-case setting in this group of patients.
Propofol was administered intravenously using a TCI pump (Diprifusor Graseby 3500). This device uses a pharmokinetic model (corrected for patient age and weight) to calculate and control infusion rates needed to achieve and maintain user set concentrations of the propofol within the blood stream, and therefore the brain. This technique is well understood by UK anaesthetists and is commonly used to provide sedation/anaesthesia for patients undergoing a variety of diagnostic and therapeutic procedures. In combination with standard doses of midazolam and fentanyl, we used a target concentration of propofol (usually 1–2 μg/ml) sufficient to obtund a conscious response to treatment while maintaining a safe cough reflex. In addition to being pleasant for the patient, the technique is reliably associated with rapid and clear-headed recovery and early discharge readiness.
Following an initial dose of intravenous paracetamol, we used a combination of propofol (titrated throughout the procedure using a TCI device as described above) and fentanyl (titrated to enhance analgesia/reflex suppression). For those cases in which we wanted to maintain a higher level of sedation reversibility we also administered an initial small dose of midazolam in order to reduce dependency on ‘non-reversible’ propofol. The level of sedation was assessed using definitions published by the National Institute for Health and Clinical Excellence (NICE) in their 2010 guidance.22 We initially aimed for ‘moderate sedation’ (NICE definition: ‘Where patients are sleepy but respond purposefully to verbal commands or light tactile stimulation’) but rapidly came to the conclusion that ‘deep sedation’ (NICE definition: ‘Patients are asleep and cannot be easily roused but do respond purposefully to repeated or painful stimulation. Patients may need assistance to maintain a patent airway’) was more appropriate to the degree of stimulation.
In addition to measuring hard quantitative endpoints, such as completion of procedure and discharge as a day case, we also looked at patient and doctor satisfaction scores as another marker of the effectiveness of the service. This demonstrated a high level of satisfaction among patients and was mirrored in the experience of both endoscopist and anaesthetist. Furthermore, there were several unmeasured benefits to using propofol, including the shorter recovery time in comparison to general anaesthesia, the ability to offer such procedures on a day-case basis, and the presence of the anaesthetist,which allowed the endoscopist to concentrate more fully on the procedure.
From an administrative prospective, it is interesting to note that since the introduction of this service, when we had no waiting list, we have seen a steep increase in demand: towards the end of this early evaluation study the median waiting time of 8 weeks was significantly greater than any of our other lists. However, what is not known is how long the wait would have been for these patients had ad-hoc operating theatre slots been required for the provision of general anaesthesia. Our experience is that now the service has become established, the demand has increased to the point at which we have had to introduce a second weekly anaesthetic assisted list.
This audit has highlighted some shortcomings of the service that need addressing, particularly with the fact that only 80% of patients listed actually had their procedure. The reason for this was twofold: (1) cancellation on the day and (2) failure to attend. The reason for the first issue was mostly due to inappropriate patient selection. In order to address this we have therefore initiated plans to set up a pre-ALPS assessment clinic (similar to pre-op assessment clinics) to assess patient suitability for deep sedation with propofol. The introduction of a policy requiring pre-assessment before allocation of a treatment appointment may also help reduce the non-attendance rate. In addition, our increase in list capacity should offer greater booking flexibility and may further reduce attendance failures.
Our additional expenditure for setting up this service included personnel, equipment and drugs/disposables costs over and above that required for a standard endoscopy service. Personnel costs included one programmed activity for both a consultant anaesthetist and an operating department practitioner, together with enhanced recovery staffing. Equipment costs would have included those for propofol infusion and capnography devices; however, we ‘borrowed’ these devices (together with disposables) from other departments within the hospital for the duration of this audit. Additional drug costs included those for propofol, ondansetron (intravenous), paracetamol (intravenous), intravenous fluids and emergency anaesthetic drugs.
We used a simple invalidated five-point score to enable us to report our preliminary findings, which is a limitation of the study. However, we are now in the process of developing a qualitative study with validated tools to allow a more sophisticated method of measuring satisfaction for a future study.
A further argument could also be made for a comparable service of providing general anaesthesia in an endoscopy suite. Indeed, a separate business case was made for an anaesthetic list in theatre for endoscopic procedures, but this was based on the preliminary data generated from the introduction of this service especially with escalating waiting times. What is important is that deep sedation is not an inexpensive alternative to general anaesthesia with tracheal intubation, but does have particular advantages in remote working with smoother recovery profile and fewer side effects for the patient, and can more easily be provided within the resources and confines of an endoscopy suite. Previous studies have demonstrated a significant reduction in patient recovery and discharge time following propofol sedation compared to general anaesthesia with tracheal intubation. This aspect is particularly attractive when considering the best method of administering deep sedation within a day-case remote endoscopy unit; however, we are in the process of collecting comparative data from these two different methods of deep sedation.
Following the start of this service we sought to audit our practice prospectively to ensure that the service was safe, effective and of high quality. With the introduction of national guidance by the BSG and the RCoA for delivering deep sedation at remote sites for complex endoscopic procedures, we were therefore able to audit our practice against established national guidance. The findings of our audit have been presented at trust level, with a re-audit planned to assess the service following the introduction of those changes identified from this audit. In addition, regular departmental quarterly service improvement meetings for anaesthetic-led propofol sedation endoscopy lists occur between gastroenterology and anaesthetics with a remit to discuss existing service delivery problems and therefore help to improve this service further.
In conclusion, propofol-based sedation for selective complex endoscopic procedures can be safely and effectively delivered for day-case patients using an ALPS within a remote endoscopy unit; and with very high patient, anaesthetist and endoscopist satisfaction scores.
What is already known in this topic?
Complex endoscopic procedures performed under propofol-based deep sedation can be associated with high rates of satisfaction for providers (anaesthetists), users (endoscopists) and consumers (patients).
What this study adds?
Propofol-based deep sedation for selective complex endoscopic procedures can be safely and effectively delivered by an Anesthetist Led Propofol Service within a day-case endoscopy unit.
Modest additional investment is required for both personnel and equipment.
A patient pre-assessment service is necessary to optimize efficiency.
Once the service is established the demand for it appears to increase rapidly.
How it might impact on clinical practice in the foreseeable future?
This model of delivery of deep-sedation for endoscopic procedures in a day-case endoscopy unit setting could be adapted to meet the ever increasing demands for an alternate and safe sedation for complex endoscopic procedures.
Footnotes
Competing interests: None.
Ethics approval: This study received ethics approval from the Royal Liverpool University Hospital Trust's clinical effectiveness and audit committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Abraham NS, Fallone CA, Mayrand S, et al. Sedation versus no sedation in the performance of diagnostic upper gastrointestinal endoscopy: a Canadian randomized controlled cost-outcome study. Am J Gastroenterol 2004;99:1692–9. [DOI] [PubMed] [Google Scholar]
- 2.Cole SG, Brozinsky S, Isenberg JI. Midazolam, a new more potent benzodiazepine, compared with diazepam: a randomized, double-blind study of pre-endoscopic sedatives. Gastrointest Endosc 1983;29:219–22. [DOI] [PubMed] [Google Scholar]
- 3.McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc 2008;67:910–23. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie N, Grant IS. Propofol for intravenous sedation. Anaesthesia 1987;42:3–6. [DOI] [PubMed] [Google Scholar]
- 5.White PF. Propofol pharmacokinetics and pharmacodynamics. Semin Anesth 1988;7:4–20. [Google Scholar]
- 6.Patterson KW, Casey PB, Murray JP, et al. Propofol sedation for outpatient upper gastrointestinal endoscopy: comparison with midazolam. Br J Anaesth 1991;67:108–11. [DOI] [PubMed] [Google Scholar]
- 7.Wehrmann T, Kokabpick S, Lembcke B, et al. Efficacy and safety of intravenous propofol sedation during routine ERCP: a prospective, controlled study. Gastrointest Endosc 1999;49:677–83. [DOI] [PubMed] [Google Scholar]
- 8.Koshy G, Nair S, Norkus EP, et al. Propofol versus midazolam and meperidine for conscious sedation in GI endoscopy. Am J Gastroenterol 2000;95:1476–9. [DOI] [PubMed] [Google Scholar]
- 9.Vargo JJ, Zuccaro G, Jr, Dumot JA, et al. Gastroenterologist administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology 2002;123:8–16. [DOI] [PubMed] [Google Scholar]
- 10.Ulmer BJ, Hansen JJ, Overley CA, et al. Propofol versus midazolam/ fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol 2003;1:425–32. [DOI] [PubMed] [Google Scholar]
- 11.Qadeer MA, Vargo JJ, Khandwala F, et al. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol 2005;3:1049–56. [DOI] [PubMed] [Google Scholar]
- 12.Riphaus A, Rabofski M, Wehrmann T. Endoscopic sedation and monitoring practice in Germany: results from the first nationwide survey. Z Gastroenterol 2010;48:392–7. [DOI] [PubMed] [Google Scholar]
- 13.Fanti L, Agostoni M, Gemma M, et al. Italian Society of Digestive Endoscopy Sedation Commission. Sedation and monitoring for gastrointestinal endoscopy: a nationwide web survey in Italy. Dig Liver Dis 2011;43:726–30. [DOI] [PubMed] [Google Scholar]
- 14.Paspatis GA, Manolaraki MM, Tribonias G, et al. Endoscopic sedation in Greece: results from a nationwide survey for the Hellenic Foundation of Gastroenterology and Nutrition. Dig Liver Dis 2009;41:807–11. [DOI] [PubMed] [Google Scholar]
- 15.Sporea I, Popescu A, Sandesc D, et al. Colonoscopy and sedation in Romania: early experience using a balanced propofol regimen. J Gastrointestin Liver Dis 2010;19:27–30. [PubMed] [Google Scholar]
- 16.Cohen LB, Wecsler JS, Gaetano JN, et al. Endoscopic sedation in the USA: results from a nationwide survey. Am J Gastroenterol 2006;101:967–74. [DOI] [PubMed] [Google Scholar]
- 17.Joint Royal College of Anaesthetists (RCoA) and British Society of Gastroenterology (BSG) Working Party. Guidance for the use of propofol sedation in adults undergoing ERCP and other complex upper GI endoscopic procedures. British Society of Gastroenterology Guidelines. 2011. http://www.bsg.org.uk/clinical-guidance/endoscopy/ (accessed June 2012).
- 18.Cotton BP, Eisen GM, Aabakken L, et al. Lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointestinal Endosc 2010;71:446–54. [DOI] [PubMed] [Google Scholar]
- 19.Dumonceau JM, Riphaus A, Aparicio JR, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates and the European Society of Anaesthesiology Guideline: non-anaesthesiologist administration of propofol for GI endoscopy. Eur J Anaesthesiol 2010;27:1016–103. [DOI] [PubMed] [Google Scholar]
- 20.Vargo JJ, Cohen LB, Rex DX, et al. Position statement: non-anaesthesiologist administration of propofol for GI endoscopy. Gastrointest Endosc 2009;70: 1053–9. [DOI] [PubMed] [Google Scholar]
- 21.Royal College of Anaesthetists. Anaesthetic services in remote sites. 2011. http://www.rcoa.ac.uk/docs/Remote-sites2011.pdf (accessed June 2012).
- 22.National Institute for Health and Clinical Excellence. Clinical Guideline 112: sedation in children and young people. NICE. 2010. http://www.nice.org.uk/guidance/CG112. (accessed June 2012). [PubMed]