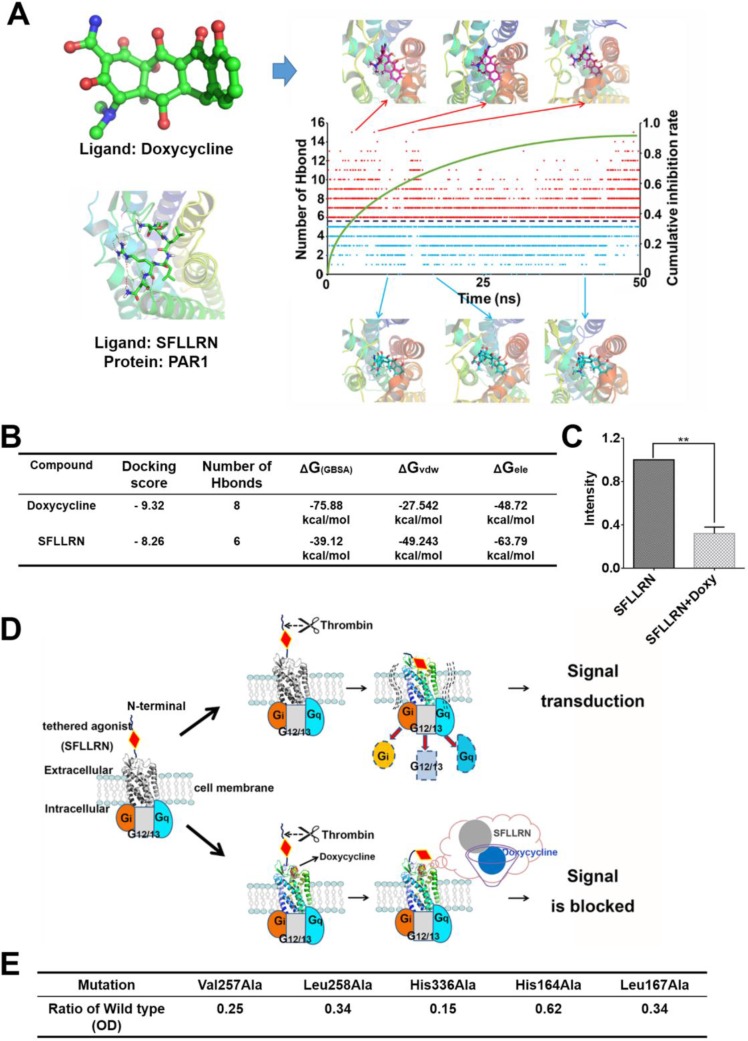
Figure 3. Molecular dynamic simulation and schematic diagram of the inhibitory mechanism showing direct interaction between doxycycline and PAR1.
(A) Changes in the number of hydrogen bonds and typical conformations between PAR1 and doxycycline in our model system. Red dots indicate that the number of hydrogen bonds was greater than six; blue dots indicate that the number of hydrogen bonds was less than six. (B) Calculated results for the binding of doxycycline and SFLLRN to PAR1, indicating that doxycycline inhibition of PAR1 is more effective than activation of PAR1 by SFLLRN. (C) Calcium signaling assay for doxycycline and SFLLRN. These results reveal that doxycycline inhibits calcium influx caused by SFLLRN. (D) The mechanism underlying doxycycline inhibition of PAR1. Doxycycline occupies the active site of PAR1 and prevents “tethered agonist” activation, ultimately blocking signal transduction. (E) Mutation of PAR1 reveal key residues involved in doxycycline binding, which is consistent with the MD results. Results were obtained from three independent experiments, each performed in triplicate, and the error bars represent the standard deviation (*P < 0.05, *P < 0.01). Data are represented as mean ± SEM.