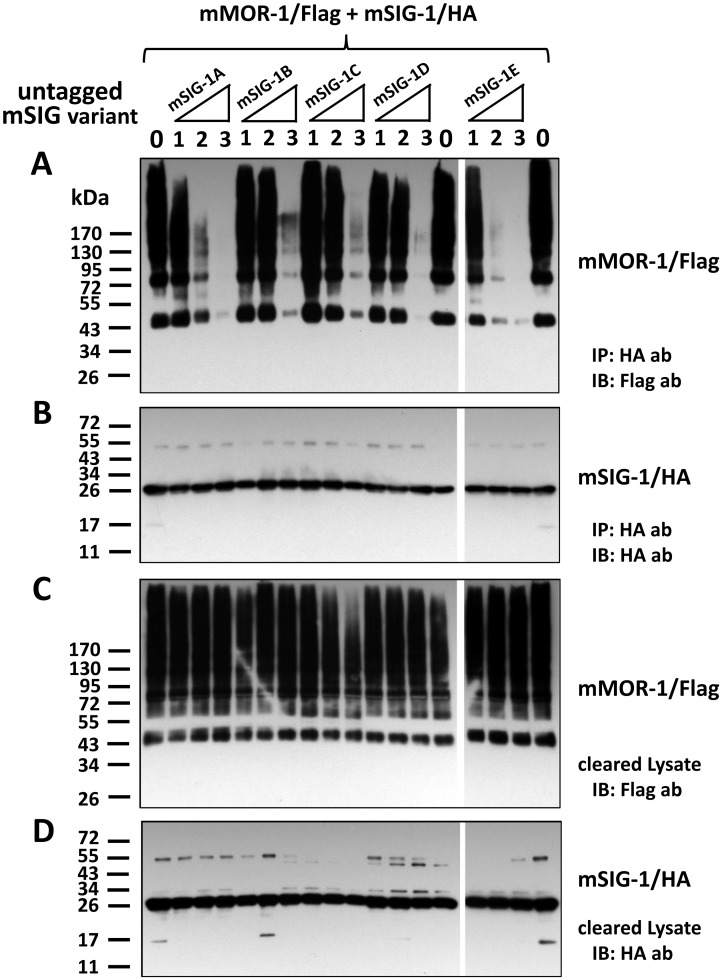
Fig 7. Effects of mSigmar1 splice variants on physical association of the original mSIG-1 with mMOR-1.
A). Western blot of HA antibody-precipitated elutes with Flag antibody (ab). Equal amounts of mSIG-1/HA (1.2 μg) and mMOR-1/Flag (1.2 μg) were co-transfected into a 100 mm plate of HEK293 cells, together with each untagged mSigmar1 variant at four different amounts (0 μg, 1.2 μg, 2.4 μg or 3.6 μg, labeled as 0, 1, 2, 3, respectively), by using Effectene (Qiagen) as described in Materials and Methods. Appropriate amount of pcDNA3 vector DNA was added to maintain the total amount of DNA in each transfection as 6 μg. Whole cells were solubilized 48 hr after transfection, and cleared lysate by centrifugation was then used in immunoprecipitation with EZview HA affinity gel. Eluted proteins by Flag peptides were separated by SDS-PAGE and transferred into a PVDF membrane, which was then immunobloted with an anti-Flag antibody, as described in Materials and Methods. The representative blot from two independent experiments was shown. IP: immunoprecipitation; IB: immunoblot. B). Western blot of HA-antibody-precipitated fraction with EZview HA affinity gel. The same elutes were analyzed using an anti-HA antibody in immunobloting. C) & D). Western blot of cleared lysate with anti-Flag antibody or anti-HA antibody. Cleared lysate samples before IP were analyzed using anti-Flag antibody (C) or an anti-HA antibody (D) in immunobloting.