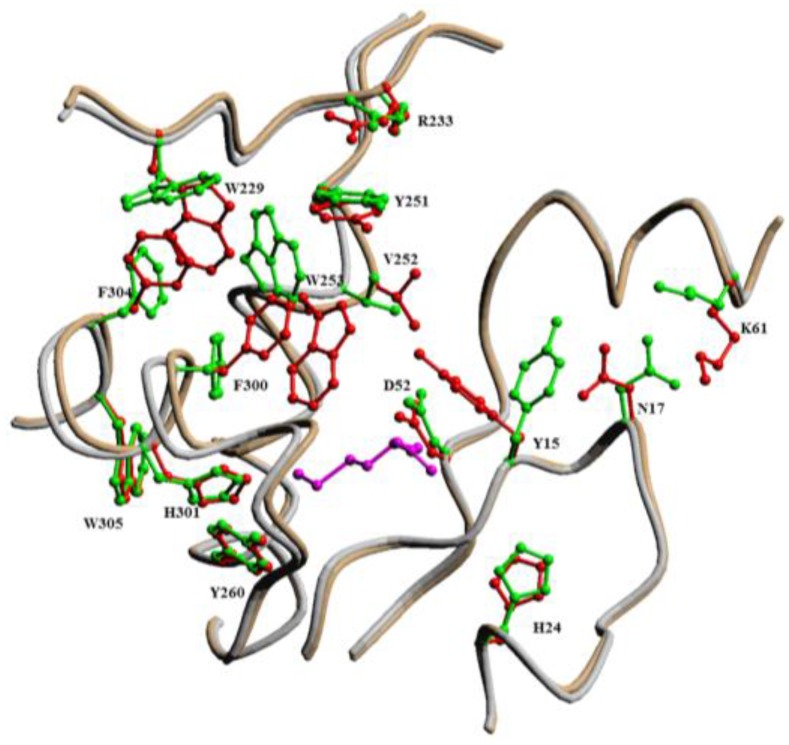
Figure 2.
The synthetic/editing active site of E. coli methionyl-tRNA synthetase (MetRS): Hydrophobic and hydrogen-bonding interactions provide specificity for the cognate substrate l-methionine. Superimposition of Cα carbon atoms for the MetRS·Met complex (beige) and free MetRS (light grey), solved at 1.8 Å resolution, shows movements of active site residues upon binding of Met. Residue colors are red in the MetRS·Met complex and green in free MetRS, and l-methionine is magenta. Reprinted with permission from ref. [57].