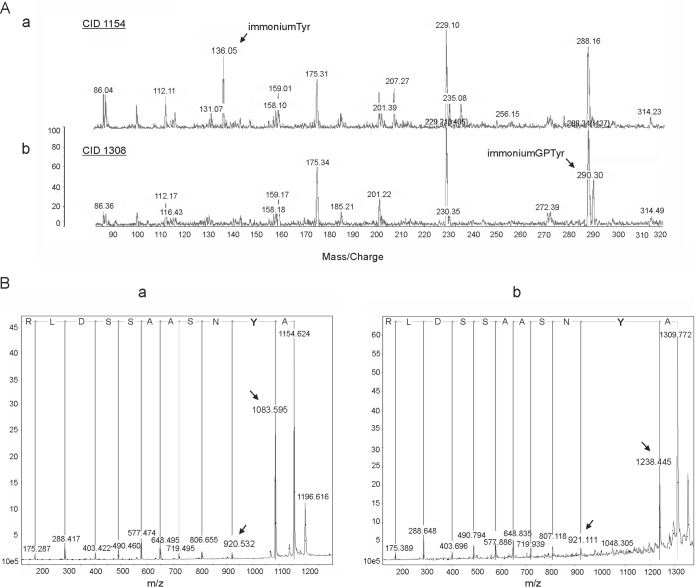
FIG 2.
Mass spectrometry analyses of the mature PilA protein of the G. sulfurreducens wild-type strain. (A) Matrix-assisted laser desorption ionization (MALDI) mass spectrometric data for the tryptic digested peptide AYNSAASSDLR of the PilA protein secreted by the wild-type G. sulfurreducens strain. Comparison of the MALDI spectra of the unmodified (a) and modified (b) AYNSAASSDLR peptide obtained by collision-induced dissociation (CID). The 1,154-Da peptide displays the unmodified tyrosine residue at 136 Da, which is not visible in the 1,308-Da peptide. The new peak emerging at 290 Da (in the 1,308-Da peptide) corresponds to a mass difference of 154 Da, suggesting a glycerophosphate-modified tyrosine. (B) MALDI-PSD (post-source decay) mass spectrometric spectra of the unmodified (a) and modified (b) AYNSAASSDLR peptide. The amino acid sequence was deduced from the spectrum on the basis of the mass difference between adjacent peaks (indicated by arrows). The mass difference of 317 Da between the peaks at 1,238 and 921 Da (b) corresponds to the combined mass of a 4-sulfophenyl-modified tyrosine residue (163 Da) (a) and a glycerophosphate group (154 Da).