Figure 1. Alteration of SR Ca cycling in the failing heart.
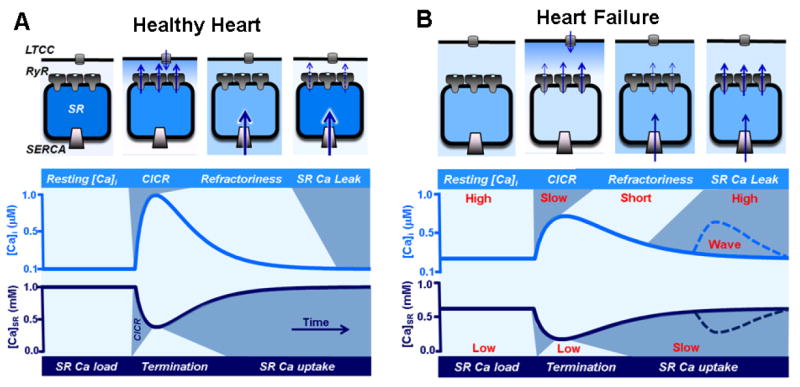
A, top: The diagram illustrates an SR Ca release unit composed by LTCCs in the T-tubule and RyR clusters in the junctional SR. SERCA pumps are predominantly located in the free SR. The activation of LTTCs during an AP increases [Ca] in the dyadic cleft. This local increase in [Ca] activates RyRs via the mechanism of CICR. Upon activation, RyRs transiently release a larger amount of Ca into the cytosol, causing to abruptly drop in [Ca]SR. The decrease in [Ca]SR deactivates RyRs, leading to termination of CICR. After SR Ca release termination, RyRs enter the refractory state which allows SERCA to replenish SR with Ca. During rest a few RyRs remain active, so the SR Ca uptake is balanced by Ca leak to finely tune the SR Ca content in preparation for the next Ca cycle. Bottom: The two traces illustrate dynamic changes of [Ca]i and [Ca]SR during ECC. All main steps of Ca cycling are marked by different colors (light and dark blue). B. In HF myocyte, the distance between LTCCs and RyRs is significantly increased. Such structural alteration decreases the fidelity of LTCC-RyR coupling causing less synchronized systolic SR Ca release. Due to the increased sensitivity of RyRs to luminal-SR [Ca], termination of systolic Ca release occurs later and at much lower [Ca]SR compared to healthy myocytes. Additionally, RyR refractoriness is shorter and RyR-mediated SR Ca leak is faster. These modifications of RyR activity lead to an enhanced propensity of pro-arrhythmic spontaneous Ca waves. Decreased SERCA activity and accelerated SR Ca leak both contribute to the depleted SR Ca content and elevated diastolic [Ca]i.
