Abstract
Agricultural residues, such as lignocellulosic materials (LM), are the most attractive renewable bioenergy sources and are abundantly found in nature. Anaerobic digestion has been extensively studied for the effective utilization of LM for biogas production. Experimental investigation of physiochemical changes that occur during pretreatment is needed for developing mechanistic and effective models that can be employed for the rational design of pretreatment processes. Various-cutting edge pretreatment technologies (physical, chemical and biological) are being tested on the pilot scale. These different pretreatment methods are widely described in this paper, among them, microaerobic pretreatment (MP) has gained attention as a potential pretreatment method for the degradation of LM, which just requires a limited amount of oxygen (or air) supplied directly during the pretreatment step. MP involves microbial communities under mild conditions (temperature and pressure), uses fewer enzymes and less energy for methane production, and is probably the most promising and environmentally friendly technique in the long run. Moreover, it is technically and economically feasible to use microorganisms instead of expensive chemicals, biological enzymes or mechanical equipment. The information provided in this paper, will endow readers with the background knowledge necessary for finding a promising solution to methane production.
Keywords: Lignocellulose, Pretreatment, Microaerobic, Microbial community, Biodegradation, Biogas
Introduction
Biomass resources are readily accessible around the world as residual wastes and agricultural biomass. The most important and abundant renewable biomass resources include crop residues, such as corn straw, wheat straw and rice straw. China has abundant biomass resources, as it is one of the largest agriculture-based economies in the world. China produces approximately 216 million metric tons of corn straw per annum, and more than half of that remains unutilized (Zhong et al. 2011). Corn straw contains non-edible plant material so called lignocellulose and is mainly composed of cellulose, hemicellulose, and lignin (Jørgensen et al. 2007). Hemicellulose is present as the matrix that surrounds the cellulose skeleton, while lignin is present as an encrusting material and serves as a protective layer. All three components have covalent cross-linkages between the polysaccharides and lignin, therefore, making biomass a composite material (Binder and Raines 2010). Anaerobic digestion (AD) is a promising method for the treatment of organic solid waste and wastewater, as it combines energy recovery with waste treatment. Lately, AD has been extensively used for treating highly biodegradable wastes, such as lignocellulosic materials, animal manure, kitchen waste and municipal sewage sludge (Qiao et al. 2013).
Pretreatment is an important tool for cellulose conversion processes, and is essential to change the structure of cellulosic biomass to make cellulose more available to the enzymes that convert the carbohydrate polymers into fermentable sugars (Mosier et al. 2005). The pretreatment step is referred to as the technological bottleneck for AD bioprocesses from LM that are cost effective. At least 20% of the total production cost is represented by the pretreatment phase in all these different approaches, thereby, making it the most expensive process step (Yang and Wyman 2008).
During the pretreatment process the compact structure of lignocellulosic is disrupted and cellulose fiber is exposed. Pretreatment of the lignocellulosic material is carried out to overcome recalcitrance through the combination of chemical and structural changes to the lignin and carbohydrates (Singh et al. 2015b). Previous studies have reported different methods of pretreatment, such as biological, chemical, mechanical and thermal process, as well as their combinations, to speed substrate hydrolysis (Wagner et al. 2013). However, according to a study, these traditional methods of pretreatment are cost intensive, as additional chemicals or energy are required (Lim and Wang 2013). Much research is needed to explore methods for lowering the cost of the conversion process. The basic understanding of each step in the process with regard to subsequent commercial viability and operation is required for commercial success in transforming biomass into energy.
Previous research has reported that hydrolysis can be enhanced by introducing a limited supply of oxygen during pretreatment or directly into the anaerobic digester (Ramos and Fdz-Polanco 2013). Microaerobic pretreatment is more economical and environmentally friendly compared to the other pretreatment methods, as it only requires a limited supply of oxygen. Previous studies have shown that microaerobic treatment has the potential to reduce the formation of toxic metabolites, such as ethanol and lactic acid, as well as facilitate the formation of certain lipids, which contribute to the stability of the anaerobe cell membrane (Lim and Wang 2013).
This paper reviews the pretreatment processes used in the production of biogas from lignocellulosic materials. The objective is to identify the strengths and weaknesses of various technologies and to find a pretreatment method suitable for industrial-scale adoption. We have identified the areas that need improvement in each of the mentioned technologies. In addition, some useful information for policy makers and researchers is given.
Pretreatment methods
Physical pretreatment
Physical pretreatment methods, including mechanical operations, different types of irradiation and ultrasonic pretreatment, have been utilized to enhance the accessibility to hydrolysable polymers within lignocellulosic material. Among the physical pretreatments, mechanical pretreatment is widely used for waste materials, such as agricultural residues or any other crops and forestry residues.
Mechanical
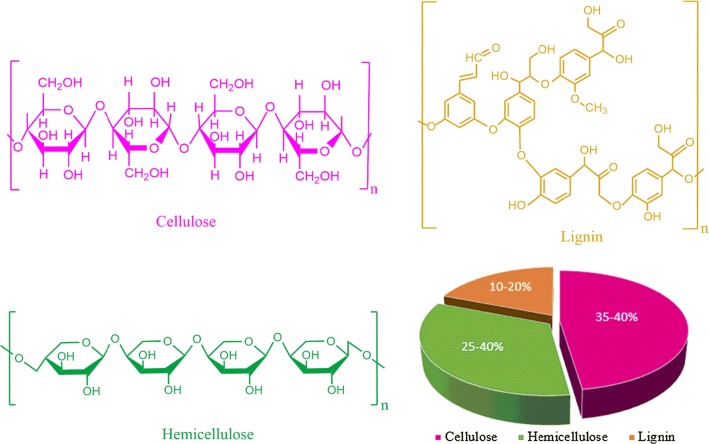
Mechanical pretreatments of lignocellulosic material is an important step for improving the bioconversion affectivity, particle densification and distribution, enzymatic accessibility, and the overall transformation of lignocellulosic material into biofuels without the generation of toxic side streams (Barakat et al. 2014). This pretreatment also generates new surface area, improves flow properties, and increases the bulk density and porosity. Lignocellulosic material has an intricate composition, as shown in Fig. 1. In mechanical comminution, different mills are used to break down the lignocellulosic material and reduce the material’s crystallinity. Commonly used mills include attrition mills, ball mills, centrifugal mills, colloid mills, hammer mills, extruders, knife mills, pin mills and vibratory mills (Cheng and Timilsina 2011).
Fig. 1.
Lignocellulose composition: cellulose, hemicellulose, and lignin
Milling reduces the crystallinity of cellulose, the substrate particle size and the degree of polymerization. The following correlation between the digestibility and structural features for wheat straw during the process of hydrolysis has been reported as shown in Eq. 1 (O’Dwyer et al. 2008).
| 1 |
The process of size reduction is energy intensive. For the proper optimization and design of biomass size-reduction equipment, the mechanical properties should be well known. The feed rate of the material, initial particle size, machine variables and moisture content greatly influence the energy requirements for reducing the size of lignocellulosic material. Fine grinding requires a large amount of energy, and there is a need to maintain a balance between efficiency improvement and cost. Thus, research efforts should be made to determine the optimal size requirements of the particle size of milled biomass.
As there is no production of inhibitors, such as furfural and hydroxyl methyl furfural (HMF), milling is best suited for both ethanol and methane production. However, this technique has a high-energy requirement and is not economically viable as a pretreatment method. Considering the high-energy requirement of milling and the sky rocketing energy prices, it is likely that milling is still not an economically viable option.
Physicochemical pretreatment
Steam explosion
Steam explosion (SE) is a well-known technique for the pretreatment of various biomass feedstocks. During SE pretreatment, lignocellulosic material is exposed to a high-pressure saturated steam at a temperature of 160–260 °C and a corresponding pressure of 5–50 atm for a few minutes. The pressure is gradually released, and the steam expands within the lignocellulosic matrix, causing individual fibers to separate and the cell wall structure to be disrupted (Kumar et al. 2009; Agbor et al. 2011). Acid can be added as a catalyst during steam explosion; however, the addition of acid is not mandatory. Steam pretreatment is termed as auto-hydrolysis if no exogenous acid catalyst is added to the plant biomass. However, more extensive lignin depolymerization can be achieved with 1% acid treatment.
Variables affecting the efficiency of SE include the moisture content, particle size, residence time and temperature (Talebnia et al. 2010). The particle size and composition of the starting material determine the relationship between the temperature and time (Viola et al. 2008). The cost of the overall process can be greatly reduced by using large particles. Decreasing the particle size of the material requires intensive mechanical comminution increases the production cost without significant increase in the sugar yield.
Hydrolysis and hemicellulose solubilization can be accomplished by either low temperature and long residence time (190 °C, 10 min) or high temperature and short residence time (270 °C, 1 min) (Duff and Murrayh 1996). The final selection of these two parameters, the residence time and temperature, is influenced by the pretreatment strategy as well as the physical accessibility and type of raw material.
Acetic acid is released as wood components are exposed to high-temperature steam, which further catalyze hydrolytic reactions of the constituent polymers. The loss of amorphous cellulose and hemicelluloses occurs as a consequence of these reactions (Martin-sampedro et al. 2011). The formation of formic and levulinic acids occur and can play a significant role in the pretreatment efficiency (Ramos 2003).
A commonly used parameter in steam pretreatment is the ‘severity factor’ (log R0), which is a measure of the severity of the pretreatment. This term combines the pretreatment temperature and the pretreatment duration in the following Eq. 2:
| 2 |
where log Ro is the severity factor (3.14–3.56 for SE) as a function of treatment time; T is the temperature in °C, where 100 °C is the reference temperature at which no solubilization occurs; t is the residence time in (min); and 14.75 is the activation energy in the current conditions, where the process obeys first-order kinetics and the Arrhenius law (Overend and Chornet 1987).
Steam explosion is effective for the pretreatment of agricultural residues and hardwoods but less effective for softwoods, where using an acid catalyst becomes significant. The cons of SE include the incomplete destruction of the lignin-carbohydrate matrix leading to the precipitation and condensation of soluble lignin components. This destroys a fragment of the xylan in hemicellulose and generates fermentation inhibitors at higher temperatures, thus making the biomass less digestible.
Microwave radiation (MWR)
The electric and magnetic field components of microwaves apply forces that rapidly change in orientation at a rate of 2.4 × 109 times per second (Galema 1997). MWR accelerates biological, chemical and physical processes due to heat and extensive collisions brought about by the vibration of polar molecules and ion movement (Sridar 1998). The performance of MWR is influenced by the dielectric properties of the lignocellulosic material. The ability of a material to store electromagnetic energy is measured by its dielectric constant, whereas the ability of a material to convert electromagnetic energy into heat is measured by its dielectric loss factor. The loss tangent (ratio of the dielectric loss factor to the dielectric constant) is calculated to measure the net efficiency of MWR.
The use of MWR-assisted biomass pretreatments has been studied, including (1) MWR/water, (2) MWR/alkali, (3) MWR/acid, (4) MWR/ionic liquid, (5) MWR/salt, and other combined MWR-assisted pretreatments (Xu 2015). MWR-assisted alkali pretreatment removes more hemicellulose and lignin from wheat straw in a shorter time, compared with traditional alkali pretreatment (Zhu et al. 2006b). Comparison of pretreatment with MWR/water, MWR/alkali and MWR/dilute acid showed that the maximum yield of total sugars after enzymatic pretreatment was attained from wheat straw pretreated by MWR/dilute acid (0.5% H2SO4, w/v) at 160 °C for 10 min, which was higher than that from MWR/alkali (0.1 g/g straw) at 160 °C for 10 min (604 mg total sugars/g straw) and MWR/water at 200 °C for 10 min (544 mg/g straw) (Saha et al. 2008). Microwave heating also accelerates cellulose dissolution in ionic liquids (Zhu et al. 2006a). The hydrolysis and MWR pretreatment of grass-type biomass into sugars was accomplished in one step by eliminating the hydrolysis step, making the process economically attractive (Marx et al. 2014). Currently, MWR is carried out on the lab scale, as the equipment is very small, and it is still difficult to apply in potential industrial projects; thus, it is not one of the most promising pretreatment methods.
Chemical pretreatment
Chemical pretreatment methods are used more often than biological or physical pretreatment methods because they are more effective and enhance the biodegradation of complex materials (Zhou et al. 2012). Common chemicals used in chemical pretreatment methods for improving the AD performance of agricultural residues are sulfuric acid (H2SO4), hydrochloric acid (HCl), acetic acid (CH3COOH), sodium hydroxide (NaOH), potassium hydroxide (KOH), lime (Ca(OH)2), aqueous ammonia (NH3∙H2O), and hydrogen peroxide (H2O2) (González et al. 2005; Us and Perendeci 2012).
Alkali pretreatment
Alkali pretreatment involves the addition of bases to biomass, leading to an increase of internal surface by swelling, a decrease of polymerization degree and crystallinity, destruction of links between lignin and other polymers, and lignin breakdown (Badiei et al. 2014). Alkali pretreatment works better for low lignin content biomass and increasing the lignin content of biomass makes this method less effective (Sun and Cheng 2002). So the effectiveness of this pretreatment depends on the lignin content of the biomass (Mudhoo 2012). NaOH, KOH and Ca(OH)2 are most reported chemicals used in alkaline pretreatment, in which process conditions are relatively mild but reaction times can be long (Harmsen et al. 2010). These pretreatments are beneficial in one way or other in accomplishing the partial hydrolysis of lignocellulosic biomasses. Up to now, NaOH and KOH are the most effective alkali-treatments for improving the biomass digestibility. According to the study, the methane yield of NaOH-pretreated corn straw was found to be approximately 220 mL/gVS, which was 73.4% higher than that of untreated corn straw as shown in Table 1. So, NaOH pretreatment has proven to be effective to improve the digestibility and increase the methane yield. However, due to concerns over sodium discharge in the process effluent that is difficult to be recycled, may limit its application on a commercial scale (Zheng et al. 2009). Though KOH could be a solution to this problem. Considering that KOH is a strong base, KOH-pretreated anaerobic digestate is gaining more importance as a fertilizer in the agriculture sector (Jaffar et al. 2016). It has been also reported that 2.5% KOH-treated CS generates maximum methane yield of 295 mL/gVS, and significantly improved 95.6% with regard to untreated CS (Li et al. 2015b). However, the high chemical loading, the toxicity to microbes, the high cost when applied in large scale, and the environmental pollution caused by the KOH is also reported (Li et al. 2015a).
Table 1.
Different pretreatment methods and their methane yields
| Pretreatment method | Pretreatment type | Pretreatment conditions | Composition changed | Gas generating capacity mL/gVS | Increased methane yield (%) | Observations | Reference |
|---|---|---|---|---|---|---|---|
| Physical methods | Mechanical pulverization | Pulverization, particle sizes of 33 to 6 mm | Cellulose, hemicellulose | – | 11–13 | Energy cost is high; particle diameter should be 6 mm for high methane yield | (Herrmann et al. 2012) |
| Physicochemical methods | Steam explosion | Pretreating silage straw 2.5 MPa, 90 s | Hemicellulose, lignin | 334.8 | 56 | Gas generating speed increased | (Guizhuan et al. 2012) |
| Microwave radiation | Frequency 2.45 GHz, power 680 W, time 24 min | Lignin | 332.3 | – | Gas generating speed is fast | (Weiwei et al. 2012) | |
| Chemical methods | H2SO4 | 2%, pretreated 7 days | Cellulose, hemicellulose | 175.6 CH4 | 74.6 | Toxic, corrosive and expensive handling | (Song et al. 2014) |
| HCl | 2%, pretreated 7 days | Cellulose, hemicellulose | 163.4 CH4 | 62.4 | Toxic, corrosive and expensive handling | (Song et al. 2014) | |
| CH3COOH | 4%, pretreated 7 days | Cellulose, hemicellulose | 145.1 CH4 | 44.2 | Toxic, corrosive and expensive handling | (Song et al. 2014) | |
| NaOH | 2%, pretreated 3 days | Hemicellulose, lignin | 220.0 CH4 | 73.4 | Toxic and hard to recycle | (Zheng et al. 2009) | |
| KOH | 2.5%, pretreated 1 day | Hemicellulose, lignin | 295.0 CH4 | 95.6 | Effective but expensive | (Li et al. 2015b) | |
| Ca(OH)2 | 2.5%, pretreated 1 day | Hemicellulose, lignin | 210.71 CH4 | 39.7 | Cheap but hard to dissolve | (Li et al. 2015b) | |
| KOH + Ca(OH)2 | 0.5 and 2%, pretreated 1 day | Hemicellulose, lignin | 271.38 CH4 | 79.9 | Cheap and effective | (Li et al. 2015b) | |
| H2O2 | 3%, pretreated 7 days | Hemicellulose, lignin | 216.7 CH4 | 115.4 | Cheap but longer pretreatment time | (Song et al. 2014) | |
| Biological methods | Mixed microorganism | XDC-2, pretreated for 16 days | Hemicellulose | 294.9 CH4 | 87. 9 | Long pretreatment time and low efficiency | (Yuan et al. 2011) |
| Adding manure | Cow dung: corn straw (1:1, w/w) pretreated for 20 days | Hemicellulose | 450.0 | 40. 7 | Highly dependent on manure type | (Zhou et al. 2012) | |
| Microaerobic pretreatment | Pretreated up to complete O2 consumption by microbes | Hemicellulose, lignin | 325.7 CH4 | 16.24 | Efficient pretreatment and cost effective | (Fu et al. 2015d) |
While, Ca(OH)2 might be better as it is low cost, safer, more environmental friendly, and can be easily recovered (Singh et al. 2015a). Ca(OH)2 has been also reported previously to enhance methane yield from lignocellulosic materials (Xiao et al. 2013). It was found that cumulative methane production of 2.5% Ca(OH)2-treated CS was found to be 210.71 mL/gVS which was 39.7% higher than that of untreated CS (Li et al. 2015b). Nevertheless, as a weak alkali, Ca(OH)2 may not improve biomass digestion significantly alone.
Some reports also focused on the combinations of two or more pretreatments to increase the biodegradability and biomethane yield during anaerobic digestion processes. Such as 0.5% KOH and 2.0% Ca(OH)2 was comparable to the effect of 2.5% KOH, obtaining a total methane yield of 271.38 mL/gVS, which was 79.9% higher than that of untreated CS as shown in Table 1 (Li et al. 2015b). However, the after effects of lime in the form of precipitate, sodium salts in the form of inhibitors, and KOH as black liquor removal and relatively high price, may limit its application (Hendriks and Zeeman 2009; Li et al. 2015b). Hence, some researchers are focusing on black liquor recycling to reduce the cost as well as the pollution (Siddhu et al. 2016).
Acid pretreatment
In addition, CH3COOH, HCl and H2SO4 pretreatments have been employed for improving the AD of lignocellulosic materials (Pakarinen et al. 2011; Monlau et al. 2013). Pretreatment with acid hydrolysis (HCl, H2SO4), can result in improvement of enzymatic hydrolysis of lignocellulosic biomass, to release fermentable sugars. Acid pretreatment results in the disruption of the van der Waals forces, hydrogen bonds and covalent bonds that hold together the biomass components, which consequently causes the solubilization of hemicellulose and the reduction of cellulose (Li et al. 2010). The main reaction that occurs during acid pretreatment is the hydrolysis of hemicellulose, especially xylan, as glucomannan is more stable. Under such conditions, furfural and HMF generation can occur, because of dehydration of xylose galactose, mannose and glucose (Hendriks and Zeeman 2009). Dilute acid hydrolysis pretreatment on the other hand can achieve high reaction rates and significantly improve cellulose hydrolysis. Lignin is hardly dissolved in most cases, but is disrupted to a high degree, thus leading to increased susceptibility of the cellulose to the enzymes (Mudhoo 2012). These pretreatments are more successful with usual concentration less than 4 wt%. Acid reagents, such as H2SO4, HCl, and CH3COOH, at concentrations of 1, 2, and 4% (w/w) have been used for pretreatment. The biodegradation of lignocellulosic straw was effectively accomplished in all pretreatments. The straw pretreated with H2SO4 (2%) and HCl (2%) acquired the highest methane yield of 175.6 and 163.4 mL/gVS among the acid pretreatments, which were 74.6 and 62.4% respectively higher than that of untreated straw, as show in Table 1 (Song et al. 2014).
For acid pretreatments, the knowledge of reaction kinetics is very important to select the suitable reactor design, configurations and operating conditions. It has been stated that hemicellulose converts to xylose by a first-order reaction with kinetic rate parameters K1 and K2 and then to furfuraldehyde and acetic acid, when biomass is exposed to a temperature higher than 180 °C (Eq. 3) (Lee et al. 1999). For the complete conversion of biomass with the high sugar and low furfural yields, hydrolysis occurs at two different stages. In the first stage, slow hydrolyzing hemicellulose at low temperature (90 °C), long retention time (50–185 min) pretreatment process with more concentrated acid (4.9–9.8%), released hemicellulosic sugars, which were then separated from biomass. While in the second stage at fast hydrolyzing hemicellulose, the remaining biomass was retreated at much higher temperature (120–130 °C) and low retention time (7–10 min) to hydrolyze cellulose to glucose. Since then, most hemicellulose hydrolysis models have been based on this reaction (Eq. 4) (Tanjore et al. 2011). However, a third variation of the basic model is the presence of an oligomeric intermediate (Eq. 5). Moreover, upon the introduction of xylo-oligomers to the kinetic analysis, the conversion of hemicellulose to soluble xylo-oligomers first occurs, which eventually converts to monomeric xylose (Jacobsen and Wyman 2000).
| 3 |
| 4 |
| 5 |
Both the inhibitor formation and the hydrolysis of lignocellulose are a function of pretreatment severity, called the combined severity factor (CSF), which is influenced by the acid concentration, reaction temperature, and retention time. Chum (Chum et al. 1990) proposed an equation to calculate the CSF based on the P-factor proposed by (Overend and Chornet 1987). These relationships are indicated in Eq. 6.
| 6 |
where the pH is the pH of the final slurry, t is the reaction time, TR is the reaction temperature, and TH is the reference temperature (100 °C).
The susceptibility of acid-pretreated biomass to cellulase treatment increases with an increase in the pretreatment severity and leads to high, nearly theoretical glucose yields. For corn straw biomass, it has been observed that with an increase in the CSF of acid pretreatment from 0.5 to 2.2, a substantially increased glucose release after enzymatic saccharification, from 32 to 57% (mass glucan/mass untreated biomass) could be achieved (Lloyd and Wyman 2005).
A feedstock pretreated with dilute acid may be slightly difficult to ferment, as fermentation inhibitors will be present. The cost of dilute acid pretreatment is higher than the other physicochemical pretreatment methods, such as AP and ammonia fiber/freeze explosion (AFEX), particularly the two-stage dilute acid pretreatment. Dilute and concentrated acids are hazardous, corrosive and toxic, and require expensive construction materials. Furthermore, acid recovery after hydrolysis leads to the secondary treatment process (Mosier et al. 2005; Kumar et al. 2009). If H2SO4 or HNO3 are used as chemical agents, formation of H2S and N2 due to reduction of sulphate and nitrate respectively, may cause a decrease in methane production (Hendriks and Zeeman 2009).
Biological pretreatment
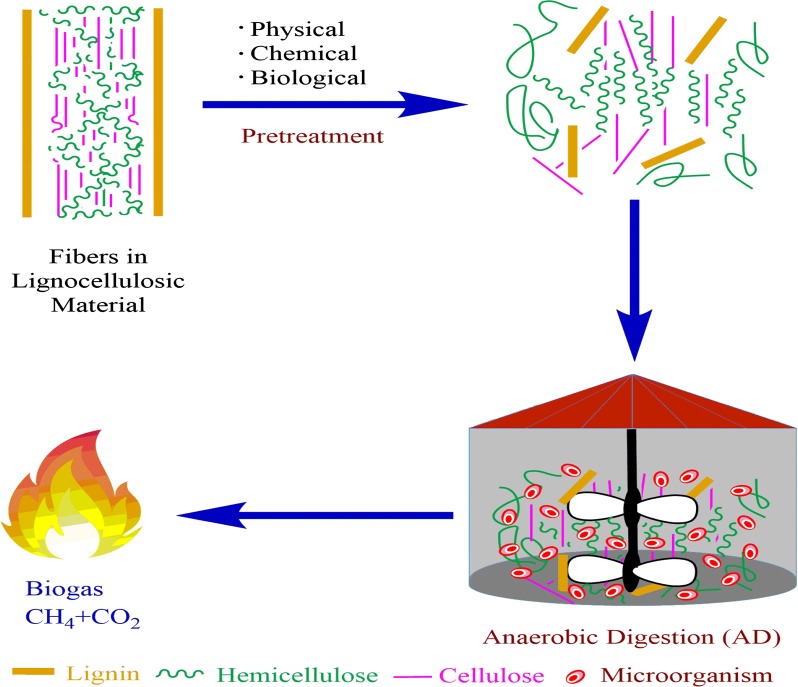
The deconstruction of lignin structures in the cell wall using microbes and/or enzymes as catalysts is usually referred to as biological pretreatment and occurs in the first stage of hydrolysis with other pretreatment processes (Tanjore and Richard 2015). The use of cellulase enzymes for converting cellulose into oligomers and sugar monomers is termed as enzymatic saccharification and occurs in the second stage of hydrolysis. Keeping these biological processes separate is conceptually convenient, but it must be considered that many of the relevant microbes simultaneously hydrolyze cellulose and lignin to obtain carbon and energy from biomass, as shown in Fig. 2. Effective biological pretreatment requires various chemical mediators and enzymes to address biochemical and physical barriers to hydrolysis; mixtures of enzymes can work synergistically for expanding small pores and increasing access by opening the cell wall matrix (Jeremic et al. 2014).
Fig. 2.
Schematic diagram of the pretreatment of lignocellulosic material for biogas production
Microbiological treatment
Bacteria such as Actinomycetes, have been observed to be effective on grasses, while fungi have gained popularity as sources of commercial plant cell wall-degrading enzymes (white-rot fungi), generating multiple cellulose-, hemicellulose- and lignin-degrading hydrolyzing enzymes. White-rot fungi have the capability to selectively metabolize low molecular weight lignin and hemicellulose while leaving cellulose relatively unaffected. Phanerochaete chrysosporium is the most well studied fungus for producing lignin-degrading enzymes. These aerobic bacteria are grown on biomass by utilizing solid-state fermentation technologies familiar to simple bench-scale laboratory systems and the mushroom industry (Saritha 2012).
The rate of biological pretreatment is very slow for industrial purposes. Some of the disadvantages of biological pretreatment that make it less suitable for industry include a long residence time of 10–14 days, extremely precise growth conditions, and the need for a large space to perform the biological pretreatment. Another potential disadvantage is that some fraction of the carbohydrate is consumed by the microorganisms. Biological pretreatment can be exploited as a first step, default pretreatment in combination with another pretreatment method or on its own if the biomass has a low lignin content (Agbor et al. 2011).
However, the most cost-effective and favorable treatments among these still need to be identified. Furthermore, the optimal concentrations for pretreatment not often reported. For the efficient and feasible utilization of agricultural residues, such information is essential.
Microaerobic pretreatment
Microaerobic pretreatment (MP) is considered to be an alternative pretreatment for the AD of corn straw in various studies. Oxygen conventionally inhibits AD. Recent studies have shown that introducing a limited supply of oxygen (or air) directly into the AD or during the pretreatment step can improve the methane yield of corn straw. The relative abundance of phylum Firmicutes, class Clostridia and order Clostridiales, which are associated with hydrolysis of AD, were grown under microaerobic conditions. Furthermore, the relative abundances of Methanobacterium and Oxytolerant were both doubled under microaerobic conditions. The reason for the improved AD performance may be the microbial community shifting under microaerobic conditions (Fu et al. 2016).
The amount of oxygen supplied during pretreatment is very important, as excessive oxygen inhibits the activity of methane-forming microorganisms and decreases the production of methane (Xu et al. 2014). In contrast, excessive oxygen can oxidize readily available substrates or facilitate aerobic Methanotrophs to consume methane. It has been also reported that thermophilic microaerobic pretreatment (TMP) before the AD of corn straw resulted in an increase in the relative abundance of phylum Firmicutes, which are associated with the production of extracellular enzymes. The relative abundance of phylum Firmicutes (especially class Bacilli, order Bacillales) was higher under microaerobic conditions than anaerobic conditions, which enables and increase in extracellular enzymes, reducing sugar, volatile fatty acids (VFAs) and soluble chemical oxygen demand (SCOD) under microaerobic condition. Therefore, the AD of corn straw was more efficient, and more methane was produced (Fu et al. 2015b).
The influence of AP and TMP on the AD of sugarcane bagasse was studied. It was seen that both AP and TMP were efficient pretreatment methods for the AD of sugarcane bagasse. The oxygen loading during TMP is of vital importance in the maximum cumulative methane production of sugarcane bagasse and can result in better crystallinity disruption, VS removal, and methane production with less lag-phase time, whereas, AP efficiently removes lignin in addition to improving the methane production rate and technical digestion time. AP requires a large amount of chemical reagent during pretreatment, whereas TMP is a cost-effective and eco-friendlier pretreatment method for the AD of sugarcane bagasse as shown in Table 2 (Fu et al. 2015a).
Table 2.
Microaerobic pretreatment of lignocellulosic biomass
| Conditions for feedstock | Conditions for inoculum | Pretreatment process | O2 Conc. (mL/gVS) | Digestion process/Temp (°C) | Gas yield (mL/gVS) | Improved yield (%) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | TS (%) | VS (%) | Type | TS (%) | VS (%) | ||||||
| Corn straw | 92.4 | 93.4 | Biogas slurry | 6.64 | 70.62 | TMP | 5 | Batch/37 | 325.7 CH4 | 16.24 | (Fu et al. 2015d) |
| Corn straw | 91.9 | 89.5 | Active sludge | 2.6 | 52.7 | TMP | 0.45/day | Batch/55 | 216.8 CH4 | 16.5 | (Fu et al. 2016) |
| Corn straw | 92.4 | 93.4 | Biogas slurry | 6.1 | 73.05 | Secondary TMT | 10 | Batch/37 | 380.6 CH4 | 28.4 | (Fu et al. 2015c) |
| Corn straw | 91.25 | 91.8 | Biogas slurry | 1.25 | 0.75 | Micro-aerobic | 0.28/day | Batch/35 | 41.6 H2 | 43 | (Li et al. 2016) |
| Sugarcane bagasse | 29.6 | 96.2 | Anaerobic sludge | 4.6 | 70.6 | TMP | 10 | Batch/37 | 229.6 CH4 | 29.28 | (Fu et al. 2015a) |
In addition to pretreatment before anaerobic digestion some researchers have suggested substrate pretreatment during the anaerobic digestion process. Secondary thermophilic microaerobic treatment (STMT) during anaerobic digestion helps in reset the digestion process by buffering the pH and increasing the microorganism activity, which provides a secondary increase in biomass degradation. This may be a successful solution to improve the low fermentation efficiency during the later stages of the anaerobic digestion process. The effect of STMT on the anaerobic digestion of corn straw improved the VS removal efficiency and afforded a higher methane yield. Similar to microaerobic pretreatment before the anaerobic digestion process, the oxygen supply in STMT during anaerobic digestion process not only reduces the concentration of toxic metabolites (e.g., ethanol, acetic acid and lactic acid) but also promotes the synthesis of certain lipids required for the stability of the anaerobe cell membrane (Fu et al. 2015c) as shown in Table 2.
Thus, TMP can be considered to be an efficient pretreatment process for AD when methane yield enhancement is a primary concern. This option has strong ability to accelerate hydrolysis, reduce the lag-phase time, and increase the methane production up to 16.24% higher than that of untreated corn digestion. The decrease in the crystallinity index, resulting from structural changes during the TMP process, may be the cause of the improvement in the methane yield of pretreated biomass as shown in Table 2 (Fu et al. 2015d).
Based upon the literature studies, it is concluded that each pretreatment method has its own merits and demerits. Although, based on feedstock types and availability of technology, the appropriate method can be selected. Among the various cutting-edge technologies, MP may be an efficient and cost effective pretreatment method that seems like a promising method and it may meet the requirements for industrial scale adoption. However, what happened during MP process is still less reported and study on whole mechanism of MP process is also lacking. Further research for technological advancement is highly recommended.
Conclusion
Pretreatment alters the various feedstock characteristics at the fiber, fibril and micro fibril level. The extent and rate of LM hydrolysis are affected by biological pretreatment, chemical pretreatment, physical pretreatment, and its morphological characteristics. However, the most cost-effective and favorable treatments among these methods have not yet been identified. Moreover, the optimal conditions for pretreatment are rarely reported. Such information is essential for the efficient and feasible utilization of different agricultural residues. One of the potential pretreatment methods reported in different studies is microaerobic pretreatment, which is more economical and environmentally friendly. MP only requires a limited amount of oxygen (or air) supplied either during a pretreatment step or directly into the anaerobic digester. The mechanism behind microaerobic pretreatment is hydrolysis initiated by the increased facultative bacteria growth rate and enzymatic activity and the greater cellulase production under microaerobic conditions. MP is an efficient and cost-effective pretreatment method that meets most of the requirements for industrial applications, such as the formation of reactive cellulosic fiber for enzymatic attack, the avoidance of the formation of possible inhibitors to the fermenting microorganisms and hydrolytic enzymes, reduced energy demand and reduced cost of size reduction of the feedstock. Other benefits include the reduction in the cost of material for construction of the pretreatment reactor and the generation of fewer residues due to zero consumption of chemicals, all of which may make MP one of the most promising and environmentally friendly techniques in the long run. At present, researchers and policy makers are in dire need of useful information that may lead to the necessary improvements in the AD industry.
Authors’ contributions
FRA collected the tabular data, created figures, and drafted the manuscript with the guidance of corresponding author (CC). Co-authors helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the financial support from Chinese Ministry of Science and Technology. This work was supported by National Key Research and Development Program of China and Teaching Reform Program in Graduate Education at Beijing University of Chemical Technology.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data are fully available without restriction.
Consent for publication
This manuscript does not contain any individual person’s data.
Ethics approval and consent to participate
No animal or human subjects were used in this work.
Funding
This study was funded by the Teaching Reform Program in Graduate Education at Beijing University of Chemical Technology (G-JG-PT201603) and the National Key Research and Development Program of China.
Abbreviations
- AD
anaerobic digestion
- AP
alkali pretreatment
- AFEX
ammonia fiber/freeze explosion
- CS
corn straw
- CSF
combined severity factor
- HMF
hydroxyl methyl furfural
- LM
lignocellulosic materials
- MC
moisture contents
- MP
microaerobic pretreatment
- MWR
microwave radiations
- SCOD
soluble chemical oxygen demand
- SE
steam explosion
- STMT
secondary thermophilic microaerobic treatment
- TMP
thermophilic microaerobic pretreatment
- TS
total solid
- VFA
volatile fatty acid
- VS
volatile solid
Contributor Information
Farrukh Raza Amin, Email: farrukhpk@mail.buct.edu.cn.
Habiba Khalid, Email: habibakhalid.khalid@gmail.com.
Han Zhang, Email: zhanghanhan2020@163.com.
Sajid u Rahman, Email: sajid_07pg30@yahoo.com.
Ruihong Zhang, Email: rhzhang@ucdavis.edu.
Guangqing Liu, Email: gqliu@mail.buct.edu.cn.
Chang Chen, Phone: +86-10-6444-2375, Email: chenchang@mail.buct.edu.cn.
References
- Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB. Biomass pretreatment: fundamentals toward application. Biotechnol Adv. 2011;29:675–685. doi: 10.1016/j.biotechadv.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Badiei M, Asim N, Jahim JM, Sopian K. Comparison of chemical pretreatment methods for cellulosic biomass. Procedia Soc Behav Sci. 2014;9:170–174. doi: 10.1016/j.apcbee.2014.01.030. [DOI] [Google Scholar]
- Barakat A, Mayer C, Solhy A, Arancon RAD, De Vries H, Luque R, Barakat A, Mayer C, Solhy A, Arancon RAD, De Vries H. Mechanical pretreatments of lignocellulosic biomass: towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014;4:48109–48127. doi: 10.1039/C4RA07568D. [DOI] [Google Scholar]
- Binder JB, Raines RT. Fermentable sugars by chemical hydrolysis of biomass. PNAS. 2010;2010:1–6. doi: 10.1073/pnas.0912073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JJ, Timilsina GR. Status and barriers of advanced biofuel technologies: a review. Renew Energy. 2011;36:3541–3549. doi: 10.1016/j.renene.2011.04.031. [DOI] [Google Scholar]
- Chum HL, Johnson DK, Black SK, Overend RP. Pretreatment-catalyst effects and the combined severity parameter. Appl Biochem Biotechnol. 1990;24(25):1–14. doi: 10.1007/BF02920229. [DOI] [Google Scholar]
- Duff SJB, Murrayh WD. Bioconversion of forest products industry waste cellulosic to fuel ethanol: a review. Bioresour Technol. 1996;55:1–33. doi: 10.1016/0960-8524(95)00122-0. [DOI] [Google Scholar]
- Fu S, Shi X, Wang F, Yuan X, Guo R-B. Comparison of thermophilic microaerobic and alkali pretreatment of sugarcane bagasse for anaerobic digestion. RSC Adv. 2015;5:63903–63908. doi: 10.1039/C5RA10717B. [DOI] [Google Scholar]
- Fu SF, He S, Shi XS, Katukuri NR, Dai M, Guo R-B. The chemical properties and microbial community characterization of the thermophilic microaerobic pretreatment process. Bioresour Technol. 2015;198:497–502. doi: 10.1016/j.biortech.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Fu SF, Shi XS, Xu XH, Wang CS, Wang L, Dai M, Guo R-B. Secondary thermophilic microaerobic treatment in the anaerobic digestion of corn straw. Bioresour Technol. 2015;186:321–324. doi: 10.1016/j.biortech.2015.03.053. [DOI] [PubMed] [Google Scholar]
- Fu SF, Wang F, Yuan XZ, Yang ZM, Luo SJ, Wang CS, Guo R-B. The thermophilic (55 °C) microaerobic pretreatment of corn straw for anaerobic digestion. Bioresour Technol. 2015;175:203–208. doi: 10.1016/j.biortech.2014.10.072. [DOI] [PubMed] [Google Scholar]
- Fu SF, Wang F, Shi XS, Guo R-B. Impacts of microaeration on the anaerobic digestion of corn straw and the microbial community structure. Chem Eng J. 2016;287:523–528. doi: 10.1016/j.cej.2015.11.070. [DOI] [Google Scholar]
- Galema SA. Microwave chemistry. Chem Soc Rev. 1997;26:233–238. doi: 10.1039/cs9972600233. [DOI] [Google Scholar]
- González G, Urrutia H, Roeckel M, Aspe E. Protein hydrolysis under anaerobic, saline conditions in presence of acetic acid. J Chem Technol Biotechnol. 2005;157:151–157. doi: 10.1002/jctb.1165. [DOI] [Google Scholar]
- Guizhuan X, Shuaiyao F, Xinfeng W, Daomeng T, Bailiang Z. Anaerobic fermentation characteristic of green corn straw pretreated by steam explosion. Trans Chin Soc Agric Eng. 2012;28:205–210. [Google Scholar]
- Harmsen P, Huijgen W, Bermudez L, Bakker R (2010) Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. Report no. 1184, pp 1–49. http://www.wur.nl/en/Publication-details.htm?publicationId=publication-way-333936323031
- Hendriks ATWM, Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresouce Technol. 2009;100:10–18. doi: 10.1016/j.biortech.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Heiermann M, Idler C, Prochnow A. Particle size reduction during harvesting of crop feedstock for biogas production I: effects on ensiling process and methane yields. Bioenergy Res. 2012;5:926–936. doi: 10.1007/s12155-012-9206-2. [DOI] [Google Scholar]
- Jacobsen SE, Wyman CE. Cellulose and hemicellulose hydrolysis models for application to current and novel pretreatment processes. Appl Biochem Biotechnol. 2000;84–86:81–96. doi: 10.1385/ABAB:84-86:1-9:81. [DOI] [PubMed] [Google Scholar]
- Jaffar M, Pang Y, Yuan H, Zou D, Liu Y, Zhu B, Korai RM, Li X. Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion ☆. Chin J Chem Eng. 2016;24:404–409. doi: 10.1016/j.cjche.2015.11.005. [DOI] [Google Scholar]
- Jeremic D, Goacher RE, Yan R, Karunakaran C, Master ER. Direct and up-close views of plant cell walls show a leading role for lignin-modifying enzymes on ensuing xylanases. Biotechnol Biofuels. 2014 doi: 10.1186/s13068-014-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen H, Kristensen JB, Felby C. Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefining. 2007;1:119–134. doi: 10.1002/bbb. [DOI] [Google Scholar]
- Kumar P, Barrett DM, Delwiche MJ, Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. 2009;48:3713–3729. doi: 10.1021/ie801542g. [DOI] [Google Scholar]
- Lee YY, Iyer P, Torget RW. Dilute-acid hydrolysis of lignocellulosic biomass. Adv Biochem Eng Biotechnol. 1999;65:94–115. [Google Scholar]
- Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M, Vogel KP, Simmons BA, Singh S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol. 2010;101:4900–4906. doi: 10.1016/j.biortech.2009.10.066. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang R, Abdul M, Siddhu H, He Y, Wang W, Li Y, Chen C, Liu G. Enhancing methane production of corn stover through a novel way: sequent pretreatment of potassium hydroxide and steam explosion. Bioresour Technol. 2015;181:345–350. doi: 10.1016/j.biortech.2015.01.050. [DOI] [PubMed] [Google Scholar]
- Li L, Chen C, Zhang R, He Y, Wang W, Liu G. Pretreatment of corn stover for methane production with the combination of potassium hydroxide and calcium hydroxide. Energy Fuels. 2015;29:5841–5846. doi: 10.1021/acs.energyfuels.5b01170. [DOI] [Google Scholar]
- Li D, Jiao C, He W, Yan Z, Yuan Y. Comparison of micro-aerobic and anaerobic fermentative hydrogen production from corn straw. Int J Hydrogen Energy. 2016;41:5456–5464. doi: 10.1016/j.ijhydene.2016.01.141. [DOI] [Google Scholar]
- Lim JW, Wang JY. Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag. 2013;33:813–819. doi: 10.1016/j.wasman.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Lloyd TA, Wyman CE. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour Technol. 2005;96:1967–1977. doi: 10.1016/j.biortech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Martin-sampedro R, Capanema EA, Hoeger I, Villar JC, Rojas OJ. Lignin changes after steam explosion and laccase-mediator treatment of eucalyptus wood chips. J Agric Food Chem. 2011;59:8761–8769. doi: 10.1021/jf201605f. [DOI] [PubMed] [Google Scholar]
- Marx S, Ndaba B, Chiyanzu I, Schabort C. Fuel ethanol production from sweet sorghum bagasse using microwave irradiation. Biomass Bioenerg. 2014;65:145–150. doi: 10.1016/j.biombioe.2013.11.019. [DOI] [Google Scholar]
- Monlau F, Latrille E, Carvalho A, Costa D, Steyer J, Carrère H. Enhancement of methane production from sunflower oil cakes by dilute acid pretreatment. Appl Energy. 2013;102:1105–1113. doi: 10.1016/j.apenergy.2012.06.042. [DOI] [Google Scholar]
- Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol. 2005;96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Mudhoo A. Biogas production: pretreatment methods in anaerobic digestion. Massachusetts: Scrivener Publishing; 2012. [Google Scholar]
- O’Dwyer JP, Zhu L, Granda CB, Chang VS, Holtzapple MT. Neural network prediction of biomass digestibility based on structural features. Biotechnol. 2008;24:283–292. doi: 10.1021/bp070193v. [DOI] [PubMed] [Google Scholar]
- Overend RP, Chornet E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos Trans R Soc London A. 1987;321:523–536. doi: 10.1098/rsta.1987.0029. [DOI] [Google Scholar]
- Pakarinen OM, Kaparaju PLN, Rintala JA. Hydrogen and methane yields of untreated, water-extracted and acid (HCl) treated maize in one- and two-stage batch assays. Int J Hydrogen Energy. 2011;36:14401–14407. doi: 10.1016/j.ijhydene.2011.08.028. [DOI] [Google Scholar]
- Qiao J, Qiu Y, Yuan X, Shi X, Xu X, Guo R. Molecular characterization of bacterial and archaeal communities in a full-scale anaerobic reactor treating corn straw. Bioresour Technol. 2013;143:512–518. doi: 10.1016/j.biortech.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Ramos LP. The chemistry involved in the steam treatment of lignocellulosic materials. Quim Nov. 2003;26:863–871. doi: 10.1590/S0100-40422003000600015. [DOI] [Google Scholar]
- Ramos I, Fdz-Polanco M. The potential of oxygen to improve the stability of anaerobic reactors during unbalanced conditions: results from a pilot-scale digester treating sewage sludge. Bioresour Technol. 2013;140:80–85. doi: 10.1016/j.biortech.2013.04.066. [DOI] [PubMed] [Google Scholar]
- Saha BC, Biswas A, Cotta MA. Microwave pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. J Biobased Mater Bioenergy. 2008;2:210–217. doi: 10.1166/jbmb.2008.412. [DOI] [Google Scholar]
- Saritha M. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J Microbiol. 2012;52:122–130. doi: 10.1007/s12088-011-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhu MAH, Li J, Zhang R, Liu J, Ji J, He Y, Chen C, Liu G. Potential of black liquor of potassium hydroxide to pretreat corn stover for biomethane production. BioResources. 2016;11:4550–4563. doi: 10.15376/biores.11.2.4550-4563. [DOI] [Google Scholar]
- Singh J, Suhag M, Dhaka A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: a review. Carbohydr Polym. 2015;117:624–631. doi: 10.1016/j.carbpol.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Singh S, Cheng G, Sathitsuksanoh N, Wu D, Varanasi P, George A, Balan V, Gao X, Kumar R, Dale BE, Wyman CE, Simmons BA. Comparison of different biomass pretreatment techniques and their impact on chemistry and structure. Front Energy Res Bioenergy Biofuels. 2015;2:1–12. doi: 10.3389/fenrg.2014.00062. [DOI] [Google Scholar]
- Song Z, Yang G, Liu X, Yan Z, Yuan Y, Liao Y. Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0093801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridar V. Microwave radiation as a catalyst for chemical reactions. Curr Sci. 1998;74:446–450. [Google Scholar]
- Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Talebnia F, Karakashev D, Angelidaki I. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol. 2010;101:4744–4753. doi: 10.1016/j.biortech.2009.11.080. [DOI] [PubMed] [Google Scholar]
- Tanjore D, Richard TL. A systems view of lignocellulose hydrolysis. In: Ravindra P, editor. Advances in bioprocess technology. Cham: Springer International Publishing; 2015. pp. 387–419. [Google Scholar]
- Tanjore D, Shi J, Wyman CE. Chapter: 4 Dilute acid and hydrothermal pretreatment of cellulosic biomass. In: Simmons B, editor. Chemical and biochemical catalysis for next generation biofuels. California: Royal Society of Chemistry; 2011. pp. 64–88. [Google Scholar]
- Us E, Perendeci NA. Improvement of methane production from greenhouse residues : optimization of thermal and H2SO4 pretreatment process by experimental design. Chem Eng J. 2012;181–182:120–131. doi: 10.1016/j.cej.2011.11.038. [DOI] [Google Scholar]
- Viola E, Cardinale M, Santarcangelo R, Villone A, Zimbardi F. Ethanol from eel grass via steam explosion and enzymatic hydrolysis. Biomass Bioenergy. 2008;32:613–618. doi: 10.1016/j.biombioe.2007.12.009. [DOI] [Google Scholar]
- Wagner AO, Schwarzenauer T, Illmer P. Improvement of methane generation capacity by aerobic pre-treatment of organic waste with a cellulolytic Trichoderma viride culture. J Environ Manage. 2013;129:357–360. doi: 10.1016/j.jenvman.2013.07.030. [DOI] [PubMed] [Google Scholar]
- Weiwei L, Huan M, Chengmao C, Zhiliang Y, Minhui Z, Xiaoling K, Xiaochen H. Effects of pretreatments with steam-explosion using solar energy and microwave irradiation on biogas production of corn stalk. Trans Chin Soc Agric Eng. 2012;28:227–234. [Google Scholar]
- Xiao X, Zhang R, He Y, Li Y, Feng L, Chen C, Liu G. Influence of particle size and alkaline pretreatment on the anaerobic digestion of corn stover. BioResources. 2013;8:5850–5860. doi: 10.15376/biores.8.4.5850-5860. [DOI] [Google Scholar]
- Xu Jian. Pretreatment of Biomass. 2015. Microwave Pretreatment; pp. 157–172. [Google Scholar]
- Xu S, Selvam A, Wong JWC. Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag. 2014;34:363–369. doi: 10.1016/j.wasman.2013.10.038. [DOI] [PubMed] [Google Scholar]
- Yang B, Wyman CE. Pretreatment : the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefining. 2008;2:26–40. doi: 10.1002/bbb. [DOI] [Google Scholar]
- Yuan X, Li P, Wang H, Wang X, Cheng X, Cui Z. Enhancing the anaerobic digestion of corn stalks using composite microbial pretreatment. J Microbiol Biotechnol. 2011;21:746–752. doi: 10.4014/jmb.1011.11026. [DOI] [PubMed] [Google Scholar]
- Zheng M, Li X, Li L, Yang X, He Y. Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour Technol. 2009;100:5140–5145. doi: 10.1016/j.biortech.2009.05.045. [DOI] [PubMed] [Google Scholar]
- Zhong W, Zhang Z, Luo Y, Sun S, Qiao W, Xiao M. Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour Technol. 2011;102:11177–11182. doi: 10.1016/j.biortech.2011.09.077. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zhang Y, Dong Y. Pretreatment for biogas production by anaerobic fermentation of mixed corn stover and cow dung. Energy. 2012;46:644–648. doi: 10.1016/j.energy.2012.07.017. [DOI] [Google Scholar]
- Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S. Dissolution of cellulose with ionic liquids and its application: a mini-review. Green Chem. 2006;8:325–327. doi: 10.1039/b601395c. [DOI] [Google Scholar]
- Zhu S, Wu Y, Yu Z, Chen Q, Wu G, Yu F, Wang C, Jin S. Microwave-assisted alkali pre-treatment of wheat straw and its enzymatic hydrolysis. Biosyst Eng. 2006;94:437–442. doi: 10.1016/j.biosystemseng.2006.04.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are fully available without restriction.