Abstract
Background
Patients with advanced diabetic kidney disease (DKD) behave differently to diabetic patients without kidney disease. We aimed to investigate the associations of hypoglycemia and outcomes after initiation of dialysis in patients with advanced DKD on dialysis.
Methods
Using National Health Insurance Research Database, 20,845 advanced DKD patients beginning long-term dialysis between 2002 and 2006 were enrolled. We investigated the incidence of severe hypoglycemia episodes before initiation of dialysis. Patients were followed from date of first dialysis to death, end of dialysis, or 2008. Main outcomes measured were all-cause mortality, myocardial infarction (MI), and subsequent severe hypoglycemic episodes after dialysis.
Results
19.18% patients had at least one hypoglycemia episode during 1-year period before initiation of dialysis. Advanced DKD patients with higher adapted Diabetes Complications Severity Index (aDCSI) scores were associated with more frequent hypoglycemia (P for trend < 0.001). Mortality and subsequent severe hypoglycemia after dialysis both increased with number of hypoglycemic episodes. Compared to those who had no hypoglycemic episodes, those who had one had a 15% higher risk of death and a 2.3-fold higher risk of subsequent severe hypoglycemia. Those with two or more episodes had a 19% higher risk of death and a 3.9-fold higher risk of subsequent severe hypoglycemia. However, previous severe hypoglycemia was not correlated with risk of MI after dialysis.
Conclusions
The rate of severe hypoglycemia was high in advanced DKD patients. Patients with higher aDCSI scores tended to have more hypoglycemic episodes. Hypoglycemic episodes were associated with subsequent hypoglycemia and mortality after initiation of dialysis. We studied the associations and further study is needed to establish cause. In addition, more attention is needed for hypoglycemia prevention in advanced DKD patients, especially for those at risk patients.
Introduction
The increasing prevalence of diabetes mellitus (DM) is a worldwide public health issue [1]. Long-term complications of DM develop gradually with time. Patients with diabetic kidney disease (DKD) make up 40–50% of patients on chronic dialysis [2]. Advanced DKD is not only a risk for hypoglycemia, but also increases the severity of hypoglycemia [3].
The Diabetes Control and Complications Trial, which tested the “Glucose Hypothesis”, found that microvascular and macrovascular disease can be reduced in patients with type 1 DM with early intensive therapy with treatment aiming for a glycated hemoglobin of 7% [4]. The United Kingdom Prospective Diabetes Study also demonstrated that using intensive therapy had long-term benefits in patients with incident type 2 DM (T2DM) [5]. However, the Action to Control Cardiovascular Risk in Diabetes Study (ACCORD) found that lowing glycated hemoglobin to 6.4% increased mortality and had no effect in reducing major cardiovascular events in T2DM patients with diabetic complication compared to those receiving standard therapy. Further analysis found intensive glycaemia control increased hypoglycaemia [6]. Retrospective epidemiological analysis of data from the ACCORD trial revealed severe hypoglycaemia increased risk of death, regardless of the intensity of glucose control [7].
Patients with advanced DKD on dialysis behave differently to DM patients without kidney disease. More than forty percent of patients with advanced DKD have severe hypoglycemia [8]. Both DKD and hypoglycemia are associated with increased morbidity and mortality [9]. Re-analysis of the ACCORD trial revealed higher risk of hypoglycemia in DKD, regardless of intensive and standard group [10]. Patients with advanced DKD usually have multiple comorbid diabetic complications. In addition, DKD is associated with an increased risk of cardiovascular (CV) events. The increase in CV risk is multifactorial: not only traditional CV risks, but also novel and uremic CV risks with kidney function deterioration. To the best of our knowledge, no study has been undertaken to evaluate the association of hypoglycemia and long-term outcomes in patients with advanced DKD. Therefore, using a large data set from the Taiwan`s National Health Insurance Research Database (NHIRD), we evaluated the association of severe hypoglycemia and outcomes after initiation of dialysis in patients with advanced DKD. Outcomes included all-cause mortality, subsequent hypoglycemia, and myocardial infarction (MI) after dialysis.
Materials and methods
Database
Since Taiwan`s National Health Insurance (NHI) program was implemented in 1995, it has provided compulsory universal health insurance to all of Taiwan`s residents with the exception of prison inmates. All medical institutions in contract with the NHI program must submit standard computerized claim documents for reimbursement of medical expenses. Patients with end-stage renal disease (ESRD) are eligible for any type of renal replacement therapy free of charge. All chronic dialysis patients are covered by NHI.
Data were obtained from Taiwan`s National Health Insurance Research Database (NHIRD) [Bureau of National Health Insurance. This database is managed by Taiwan`s National Health Research Institute who released the data to researchers. The NHIRD, which contains data from nearly all (99%) inpatient and outpatient medical benefit claims made for Taiwan’s 23 million residents, is one of the largest and most comprehensive databases in the world, and has been used extensively in various studies [available at http://nhird.nhri.org.tw/ (In Chinese) and http://nhird.nhri.org.tw/en/How_to_cite_us.html (In English)]. Gender, birthday, dates of admission and discharge, medical institutions providing the services, diagnostic and procedure codes (up to five each), and outcomes are encrypted. This study tapped the NHIRD to gain access to ambulatory care claims data, inpatient claims, the Catastrophic Illness Database (CID) and updated beneficiary registry data. As the dataset was released with deidentified secondary data for public research purposes, the study was exempt from full review by the Institutional Review Board of Chi-Mei medical center and the Bureau of National Health Insurance (NHRINHIRD-99182).
In Taiwan, the NHI Bureau issues major illness/injury certificates to all patients who had ESRD needed received long-term dialysis. So, ESRD needed received long-term dialysis is among the listed catastrophic illness in the CID in Taiwan. Individuals who are registered in the CID for ESRD needed received long-term dialysis must provide the NHI Administration review board a physician`s diagnosis certificate and relevant medical records, including dialysis information. ESRD need received long-term dialysis was validated through an expert review process. When the applications are approved, the patients are exempted from disease-related copayments for medical services, including the dialysis fees.
Patient selection and definition
For this longitudinal cohort study, we first identified adult ESRD patients (≥18 years old) on maintenance dialysis beginning between January 1, 2002 and December 31, 2006. Maintenance dialysis was defined as having received dialysis for more than 90 days. A total of 39,956 incident ESRD dialysis patients were identified. Sixty-seven patients without birth date information were excluded. The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes used to define DM: 250.xx, 357.2, 362.0x, 366.41. Patients with DM were identified according to one of the definitions below: (1) diagnostic codes from outpatient visits if the patient had an initial diagnosis at any time in the 1 year before the start of dialysis and then had one or more additional diagnoses within the subsequent 12 months. The first and last outpatient visit within 1 year had to have been >30 days apart to avoid accidental inclusion of miscoded patients; (2) diagnostic codes in hospitalization databases at least once in the 1 year before the start of dialysis. Among the remaining 39, 889 incident ESRD dialysis patients, 20,883 had DM. Next, patients who had undergone renal transplantation before beginning dialysis (n = 38) were excluded. Finally, this study analyzed data collected from 20, 845 adult advanced DKD patients needed long-term dialysis. Severe hypoglycemia was defined as requiring medical assistance; these patients were identified by a listing of one of the following ICD-9-CM codes: 250.8 (diabetic hypoglycemia, hypoglycemic shock), 251.0 (hypoglycemic coma), 251.1 (hyperinsulinism hypoglycemia) or 251.2 (unspecified hypoglycemia) in the emergency department or inpatient databases any time during the one-year period leading up to beginning of dialysis. We also collected patient demographic data, survival status, date of death, adapted Diabetes Complications Severity Index (aDCSI) score [11–13] and baseline comorbidities.
Outcomes
The following outcomes were considered: all-cause mortality, subsequent severe hypoglycemia on dialysis within the one-year period following initiation of dialysis, and MI. Antecedent hypoglycemia can blunt subsequent counterregulatory response to hypoglycemia in a short-term period [14]. Moreover, previous hypoglycemia is a risk factor of subsequent hypoglycemia. Yun et al. reported 22.4% of patients with severe hypoglycemia experienced previous hypoglycemic events within 3 months [15]. Some evidences showed hypoglycemia in the previous 6 months was associated with recurrent hypoglycemia in T2DM patients [16, 17]. HYPOS-1 study demonstrated 16.5% T1DM patients experienced severe hypoglycemia in the past 12 months and 78.6% patients had at least one hypoglycemia in the past 4 weeks [18]. To our best of knowledge, no longer interval was studied in the literature. Therefore, we choose 1-year period to investigate the relationship of previous hypoglycemia and subsequent episode. Patients were followed-up from the first reported date of dialysis to the date of death, end of dialysis, or December 31, 2008, whichever occurred first.
Statistical analyses
Baseline characteristics between the groups who had hypoglycaemic episodes and those who had not were compared using a parametric Pearson’s chi-square test. Significance was set at p < 0.05. The cumulative proportion of survivors after the initiation of dialysis were calculated using the Kaplan-Meier method. The log rank test was used to analyze significance. Cox proportional hazards models were used to identify the risk factors of mortality after the initiation of dialysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were derived from Cox proportional hazards models. Cox models met the assumption of proportionality of risks. The independent associations were examined using multivariate analysis. All statistical operations were performed using the Statistical Package for Social Sciences for Windows 17.0 (SPSS Inc; Chicago, IL, USA).
Results
Patient characteristics
We enrolled 20,845 adult advanced DKD patients started receiving dialysis between 2002 and 2006. Among these patients, 19849 (95.2%) received hemodialysis (HD) and 996 (4.8%) received peritoneal dialysis (PD). Of these patients, 16,847 had no episode of severe hypoglycemia at any time within 1-year period before starting dialysis, 3,019 (14.48%) patients had one episode of severe hypoglycemia, and the other 979 (4.70%) patients had 2 or more episodes (Table 1). Female patients had a higher incidence of hypoglycemia than male patients. Baseline comorbidities included congestive heart failure (CHF), coronary artery disease (CAD), cerebrovascular accident (CVA), dysrhythmia, other cardiac diseases, peripheral vascular disease (PVD), chronic obstructive lung disease (COPD), gastrointestinal (GI) bleeding, liver disease and cancer, all factors relevant to survival in ESRD patients on dialysis [19, 20]. Because all of our patients were advanced DKD on maintenance dialysis, the minimum of aDCSI score was 2. Advanced DKD patients with higher aDCSI scores tended to have a higher frequency of hypoglycemia (P for trend < 0.001). 9.4%, 11.6%, 12.2%, and 17.3% of patients with aDCSI scores 2, 3, 4, and ≥ 5, respectively, had one episode of severe hypoglycemia. In addition, 2.1%, 3.1%, 3.3%, and 6.3% of the patients with aDCSI scores 2, 3, 4, and ≥ 5 had two or more episodes (p< 0.001). Patients on HD had a higher incidence of severe hypoglycemia with 1-year before starting dialysis than those on PD (P<0.001). 3850 (19.4%) of patients on HD and 148 (14.9%) of those on PD had severe hypoglycemia episodes. Patients with severe hypoglycemia tended to have more comorbidities, particularly CHF, CVA, COPD and GI bleeding.
Table 1. The baseline characteristics of diabetic end-stage renal disease dialysis patients by hypoglycemic episodes status.
| Without Hypoglycemia (n = 16847) |
With 1 hypoglycemic episode (n = 3019) |
With ≥2 hypoglycemic episodes (n = 979) |
P | ||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Sex | <0.001 | ||||||
| Female | 8257 | (79.5) | 1573 | (15.1) | 553 | (5.3) | |
| Male | 8590 | (82.1) | 1446 | (13.8) | 426 | (4.1) | |
| Age (years) | 0.05 | ||||||
| 18–44 | 1068 | (80.8) | 172 | (13) | 82 | (6.2) | |
| 45–64 | 8079 | (80.8) | 1451 | (14.5) | 470 | (4.7) | |
| ≥ 65 | 7700 | (80.9) | 1396 | (14.7) | 427 | (4.5) | |
| aDCSI score | <0.001 | ||||||
| 2 | 2440 | (88.4) | 260 | (9.4) | 59 | (2.1) | |
| 3 | 1935 | (85.3) | 264 | (11.6) | 70 | (3.1) | |
| 4 | 3952 | (84.5) | 573 | (12.2) | 153 | (3.3) | |
| ≥ 5 | 8520 | (76.5) | 1922 | (17.3) | 697 | (6.3) | |
| Congestive heart failure | <0.001 | ||||||
| No | 10007 | (82.2) | 1677 | (13.8) | 493 | (4) | |
| Yes | 6840 | (78.9) | 1342 | (15.5) | 486 | (5.6) | |
| Coronary artery disease | 0.114 | ||||||
| No | 9799 | (81.2) | 1738 | (14.4) | 537 | (4.4) | |
| Yes | 7048 | (80.4) | 1281 | (14.6) | 442 | (5) | |
| Cerebrovascular disease | <0.001 | ||||||
| No | 12559 | (82) | 2094 | (13.7) | 664 | (4.3) | |
| Yes | 4288 | (77.6) | 925 | (16.7) | 315 | (5.7) | |
| Dysrhythmia | 0.109 | ||||||
| No | 15006 | (80.6) | 2712 | (14.6) | 890 | (4.8) | |
| Yes | 1841 | (82.3) | 307 | (13.7) | 89 | (4) | |
| Other cardiaca | 0.349 | ||||||
| No | 14295 | (80.9) | 2562 | (14.5) | 814 | (4.6) | |
| Yes | 2552 | (80.4) | 457 | (14.4) | 165 | (5.2) | |
| Peripheral vascular disease | 0.349 | ||||||
| No | 15314 | (81.1) | 2699 | (14.3) | 859 | (4.6) | |
| Yes | 1533 | (77.7) | 320 | (16.2) | 120 | (6.1) | |
| Chronic obstructive lung disease | <0.001 | ||||||
| No | 13876 | (81.4) | 2426 | (14.2) | 747 | (4.4) | |
| Yes | 2971 | (78.3) | 593 | (15.6) | 232 | (6.1) | |
| Gasrointestinal bleeding | <0.001 | ||||||
| No | 10749 | (81.6) | 1858 | (14.1) | 567 | (4.3) | |
| Yes | 6098 | (79.5) | 1161 | (15.1) | 412 | (5.4) | |
| Liver disease | 0.05 | ||||||
| No | 14321 | (81) | 2563 | (14.5) | 804 | (4.5) | |
| Yes | 2526 | (80) | 156 | (14.4) | 175 | (5.5) | |
| Cancer | <0.001 | ||||||
| No | 15636 | (80.5) | 2842 | (14.6) | 934 | (4.8) | |
| Yes | 1211 | (84.5) | 177 | (12.4) | 45 | (3.1) | |
aDCSI = adapted Diabetes Complications Severity Index;
a Includes pericarditis, endocarditis, myocarditis, other complications of heart disease, heart transplant, heart valve replacement, and cardiac devices.
Risk factors for all-cause mortality following the initiation of dialysis
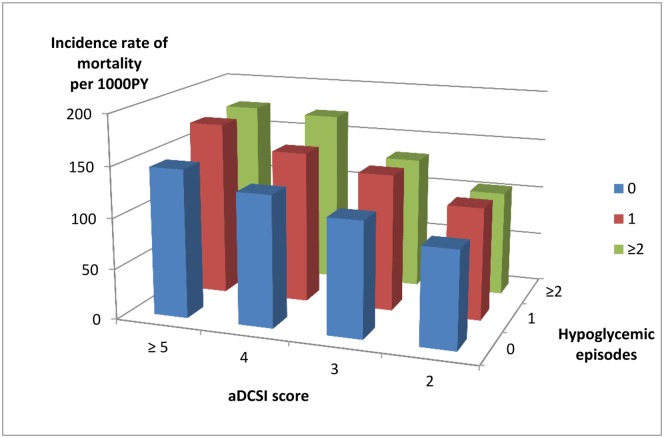
During a mean follow-up period of 3.08 ± 1.67 years after dialysis, 8,732 (41.9%) had died. We stratified these DKD patients by aDCSI score and hypoglycemic episodes to investigate their influence on clinical outcomes. Mortality tended to rise along with increase in number of previous hypoglycemic episodes and increases in aDCSI scores (Fig 1). Among patients that had not previously severe hypoglycemia episode, 791 (32.4%), 722 (37.3%), 1585 (40.1%) and 3760 (44.1%) of those with aDCSI scores 2, 3, 4, and ≥ 5 died of any cause. Among those that had one episode of severe hypoglycemia, 91 (35%), 115 (43.6%), 256 (44.7%) and 944 (49.1%) of those with aDCSI scores 2, 3, 4, and ≥ 5 died of any cause. Among those that had two or more episodes of severe hypoglycemia, 20 (33.9%), 30 (42.9%), 73 (47.7%) and 345 (49.5%) of those with aDCSI scores 2, 3, 4, and ≥ 5 died of any cause.
Fig 1. Incidence of mortality after the initiation of dialysis stratified by adapted Diabetes Complications Severity Index (aDCSI) score and hypoglycemic episodes.
After adjustment, patients had severe hypoglycemia episodes were at a higher risk of mortality (Table 2). Compared to those with no previous severe hypoglycemic episodes, those with one episode had a 15% higher death risk and those with two or more had a 19% higher death risk. Mortality risk also increased 10% with each 1-point increment in aDCSI score.
Table 2. Risk factor for all-cause mortality, subsequent severe hypoglycemia on dialysis (within 1-year period after the initiation of dialysis), and Myocardial Infarction (MI).
| Covariate | HR (95% CI) for all-cause mortality | HR (95% CI) for Subsequent severe hypoglycemia | HR (95% CI) for myocardial infarction |
|---|---|---|---|
| Sex (Male v Female) | 1.080 (1.035–1.125)* | 0.989 (0.908–1.078) | 1.108 (0.994–1.234) |
| Age (year; each increment of 1 year) | 1.041 (1.039–1.043)* | 1.001 (0.997–1.005) | 1.024 (1.019–1.030)* |
| aDCSI score (each increment of 1 score) | 1.104 (1.092–1.116)* | 1.117 (1.094–1.140)* | 1.135 (1.105–1.166)* |
| Hypoglycemic episodes | |||
| No | 1 | 1 | 1 |
| 1 | 1.146 (1.082–1.215)* | 2.279 (2.060–2.522)* | 1.065 (0.918–1.237) |
| ≥ 2 | 1.194 (1.186–1.312)* | 3.903 (3.429–4.441)* | 0.904 (0.691–1.182) |
* HR adjusted for sex, age, hypoglycemic episodes, aDCSI score and extra—comorbidities (except comorbidities in aDCSI score, including chronic obstructive lung disease, gasrointestinal bleeding, liver disease and cancer).
aDCSI = adapted Diabetes Complications Severity Index
Risk factors for severe hypoglycemia within 1-year following the initiation of dialysis
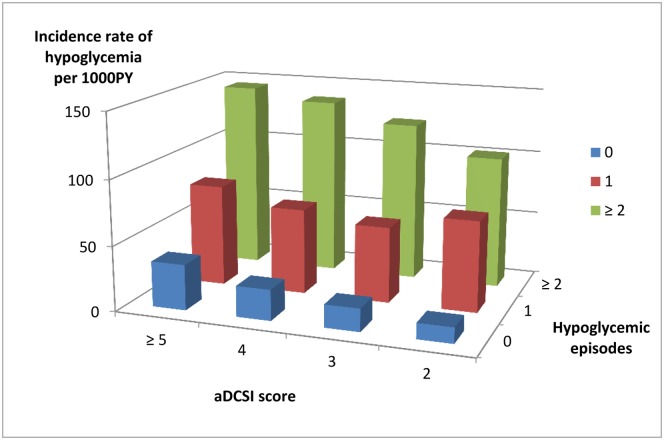
We studied the incidence of subsequent severe hypoglycemic episodes within 1-year following the initiation of dialysis. The incidence rate of subsequent severe hypoglycemia on dialysis increased along both with increases in previous hypoglycemic episodes and increases in sDCSI scores (Fig 2). In patients that had not previously severe hypoglycemic episode, 100 (4.1%), 107 (5.5%), 274 (6.9%) and 837 (9.8%) of those with aDCSI scores 2, 3, 4, and ≥ 5 had a subsequent severe hypoglycemic episode within 1-year following the initiation of dialysis. Among those who had one severe hypoglycemic episode, 49 (18.8%), 43 (16.3%), 97 (16.9%) and 355 (18.5%) of those with aDCSI scores 2, 3, 4, and ≥ 5 had a subsequent severe hypoglycemic episode. Of those who had two or more severe hypoglycemic episodes, 15 (25.4%), 21 (30%), 45 (29.4%) and 211 (30.3%) of those with aDCSI scores 2, 3, 4, and ≥ 5 had subsequent severe hypoglycemia on dialysis. We further analyzed different dialysis modalities and hypoglycemia. Patients on HD had a higher incidence of severe hypoglycemia within 1-year following the initiation of dialysis; 2078 (10.5%) of patients on HD and only 76 (7.6%) of those on PD had severe hypoglycemia episodes (P<0.004).
Fig 2. Incidence of subsequent severe hypoglycemia on dialysis (within 1-year period after the initiation of dialysis) stratified by adapted Diabetes Complications Severity Index (aDCSI) score and hypoglycemic episodes.
After multivariate adjustment, patients with previous severe hypoglycemia episodes were found to be at a higher risk of subsequent severe hypoglycemia on dialysis (Table 2). Compared to those with no previously severe hypoglycemic episodes, those with one episode had a 2.28-fold higher subsequent severe hypoglycemia risk and those with 2 or more a 3.90-fold higher subsequent severe hypoglycemia risk. Subsequent severe hypoglycemia risk also increased 12% with each increment of 1 aDCSI score.
Risk factors for subsequent myocardial infarction following the initiation of dialysis
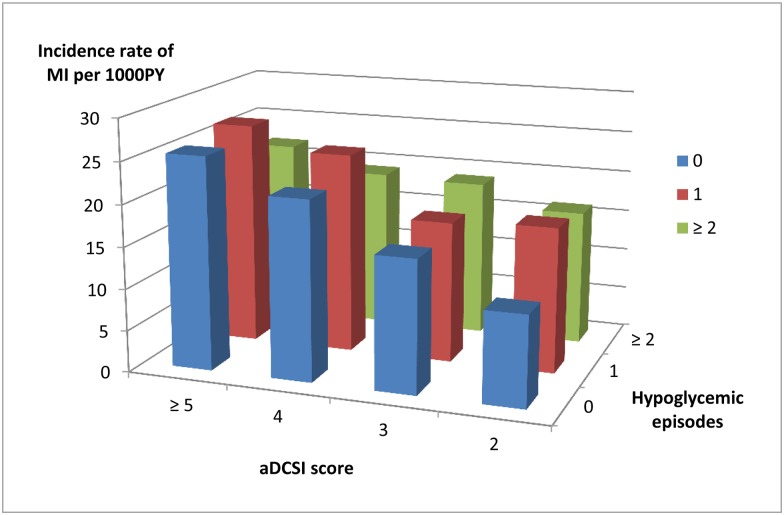
We analyzed the risk for subsequent MI in DKD patients who had begun dialysis. Among those who had no previously severe hypoglycemic episode, 89 (3.6%), 99 (5.1%), 258 (6.4%) and 636 (7.5%) of patients with aDCSI scores 2, 3, 4, and ≥ 5 patients had a MI on dialysis. Among those who had one episode of severe hypoglycemia, 14 (5.4%), 14 (5.3%), 40 (7%) and 140 (7.4%) of patients with aDCSI scores 2, 3, 4, and ≥ 5 had a MI on dialysis. Among those with two or more episodes of severe hypoglycemia, 3 (5.1%), 4 (5.7%), 8 (5.2%) and 42 (6.0%) of patients with aDCSI scores 2, 3, 4, and ≥ 5 had a MI on dialysis (Fig 3).
Fig 3. Incidence of myocardial infarction after the initiation of dialysis stratified by adapted Diabetes Complications Severity Index (aDCSI) score and hypoglycemic episodes.
After multivariate adjustment, patients who had severe hypoglycemia episodes previously were not found to be at greater risk of MI on dialysis (Table 2). However, the risk of MI increased 14% with each one point increment in aDCSI score (HR: 1.14, 95% CI: 1.11–1.17).
Discussion
This is the first national cohort study to evaluate the associations of severe hypoglycemia and outcomes after dialysis in advanced DKD patients. DM Patients with advanced kidney disease behave differently to those without kidney disease. We found 19.18% of advanced DKD patients had at least one episode of hypoglycemia the year leading up to the beginning of their dialysis therapy. Advanced DKD patients with higher aDCSI scores tended to have more frequent episodes of hypoglycemia. The number of severe hypoglycemic episodes was associated with risk of mortality and subsequent hypoglycemia after dialysis. While, subsequent MI after dialysis was not associated with previous hypoglycemic episodes.
Patients with advanced DKD have a higher risk of hypoglycemia. In another study, only 2% of 15,404 DM patients had at least one episode of hypoglycemia in a 3-year period [21]. The ADVANCE study reported that 2.7% patients in an intensive-control group and 1.5% patients in a standard-control group had at least one severe hypoglycemic episode during a median follow-up of 5 years [22]. The ACCORD study reported that 16.2% and 5.1% of the patients in an intensive therapy group and a standard therapy group, respectively, had hypoglycemia requiring assistance during a mean follow up of 3.5 years [6]. The current study also found DKD patients with higher aDCSI scores tended to have more frequent episodes of hypoglycemia. Hypoglycemia may indicate an underlining health problem [23]. The incidence of hypoglycemia is higher in patients with DKD than those without DKD [3], probably due to the reduction in insulin requirement associated with renal insufficiency [24]. Furthermore, advanced DKD patients have been found to be at greater risk of hypoglycemia in cases involving decreased degradation of insulin in peripheral tissue [25], anorexics with suboptimal nutrition [26], reduced renal gluconeogenesis, and prolonged half-life of antidiabetic drugs. Rates of hypoglycemia have been found to be higher among those with older age, CHF, CAD, CVA, renal failure, autonomic neuropathy, and adrenocortical insufficiency [27–29]. In the current study, older patients and female patients with more comorbidities had higher rates of hypoglycemia.
This study found hypoglycemia to be associated with higher mortality risk in patients with advanced DKD. Hypoglycemia has been reported to be related to increases in one-day mortality in patients with DKD [3]. The ADVANCE study reported severe hypoglycemia to be associated with an increase in death risk [22]. Furthermore, we found an association between frequency of hypoglycemic episodes and increased long-term mortality in ESRD dialysis patients. It is controversial whether the hypoglycemia causes death or whether it is a predictor of underlying problems and poor outcome. After adjustment of aDCSI scores, we found risk of mortality on dialysis increased along with both number of hypoglycemic episodes and increased in aDCSI score. Thus, based on our results, hypoglycemia in DKD patients may not only be a marker of poor outcome, it may also be associated with death. However, because we studies associations, further study is needed to establish cause.
This study found hypoglycemic episodes in DKD to be associated with subsequent hypoglycemia within 1-year following the initiation of dialysis. Previous hypoglycemia is a risk factor associated with further hypoglycemia [30]. We also found increases in aDCSI score to be associated with subsequent hypoglycemia on dialysis. CAD, infection, and poor renal function have been reported to be associated with risks of recurrent hypoglycemia [31]. The HYPOS-1 study reported the rate of hypoglycemia was higher in patients with previous severe hypoglycemia, neuropathy, long duration, and on polypharmacy [18]. Therefore, patients with more comorbidies are at risk for recurrent hypoglycemia and should be closely monitored.
In Taiwan, 95.2% of DM patients with ESRD received HD and only 4.8% received PD. Glucose is one of the contents of hemodialysates [32] and peritoneal dialysates [33]. Different dialysis modalities have different glucose-added dialysates, which maybe an important factor to influence blood glucose. Glucose-added hemodialysates (commonly 100 to 200 mg per deciliter) significantly reduced hypoglycemia in ESRD receiving HD [34, 35]. In addition, higher hemodialysate glucose concentration significantly reduced more hypoglycemia compared with lower hemodialysate glucose concentration [36, 37]. The glucose concentrations of peritoneal dialysates are much higher than those of hemodialysates. In addition, ESRD patients on PD, who received 24-hour continue high-glucose-concentration peritoneal dialysates, can develop hyperglycemia and transient hyperinsulinism [38]. Chinese patients in Hong Kong have a high prevalence of hyperglycemia with daily exchange of 1.5% glucose dialysate [39]. Frequency of hypoglycemia was higher in patients on HD than those on PD [40]. In the current study, we had similar finding. We found 10.5% of DM patients on HD and only 7.6% of those on PD had severe hypoglycemia episodes within 1-year following the initiation of dialysis.
It has been demonstrated hypoglycemic episodes increase CV risk in general population [41–44]. In the current study, we found hypoglycemic episodes in DKD did not correlate with subsequent MI on dialysis. The lack of correlation may be explained by different pathways resulting in CVD in advanced DKD patients. The mechanisms through which DKD may promote CVD include traditional cardiovascular risk factors in general population (e.g., age, male gender, hyperglycemia, DM, hypertension, and smoking) and nontraditional risk factors (e.g., accumulation of uremic toxins, anemia, and disordered mineral metabolism, vascular calcification, hyperhomocysteinemia, inflammation, and oxidative stress) [45, 46].
This study has some limitations. One limitation is that we used ICD-9-CM code to identify various diseases in this study, and thus there is a possibility of misclassification. Additionally, our data were obtained from the NHIRD, a database of insurance claims, which means it lacks laboratory data such as glucose level or glycated hemoglobin. Finally, polypharmacy and anti-diabetic agents are identified as risk factors for hypoglycemia. In HYPOS-1 study, polypharmacy was associated with a 24% excess of risk for hypoglycemia [18]. Hypoglycemia episodes were higher among those with insulin and insulin secretagogues [15, 27]. In addition, angiotensin-converting enzyme inhibitors and beta blockers may contribute to precipitating hypoglycemia by masking the warning symptoms [18]. Therefore, it would be better to describe the data on medications received by subjects; however, there was no information of medications in our database. Further studies needed to be performed to evaluate it.
In conclusion, the rate of severe hypoglycemia was high in advanced DKD patients. Severe hypoglycemic episodes and diabetic complications associated with subsequent hypoglycemia and mortality in these patients once on dialysis. We studied the associations and further study is needed to establish cause. In addition, more attention is needed in the monitoring of glucose and the use of anti-diabetic medications in DKD patients, especially for those at risk patients.
Abbreviations
- aDCSI
adapted Diabetes Complications Severity Index
- CAD
coronary artery disease
- CHF
congestive heart failure
- COPD
chronic obstructive lung disease
- CV
cardiovascular
- CVA
cerebrovascular accident
- DKD
diabetic kidney disease
- DM
diabetes mellitus
- ESRD
end-stage renal disease
- MI
myocardial infarction
- NHIRD
National Health Insurance Research Database
- PVD
peripheral vascular disease
- T2DM
Type 2 diabetes mellitus
Data Availability
Data are available from the National Health Insurance Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Contact information for the National Health Insurance Research Data Access: Center for Biomedical Resources of NHRI, 35 Keyan Road, Zhunan, Miaoli County 35053, Taiwan. Tel: 037-246166ext.33603 eMail: nhird@nhri.org.tw.
Funding Statement
The study was supported by grant CMFHR10267 for Dr. Yeh-Wen Chu and CMFHR10543 for Dr. Chih-Chiang Chien from Chi-Mei Medical Center and grant NHRI-NHIRD-99182 from the National Health Research Institutes in Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:A7, e1–420. [DOI] [PubMed] [Google Scholar]
- 3.Moen MF, Zhan M, Hsu VD, Walker LD, Einhorn LM, Seliger SL, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1121–7. 10.2215/CJN.00800209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, et al. ; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. 10.2337/dc13-2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 6.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA,et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909 10.1136/bmj.b4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K, et al. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27:135–145. 10.1111/sdi.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsahli M, Gerich JE, et al. Hypoglycemia, chronic kidney disease, and diabetes mellitus. Mayo Clin Proc. 2014;89:1564–1571. 10.1016/j.mayocp.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 10.Papademetriou V, Lovato L, Doumas M, Nylen E, Mottl A, Cohen RM, et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649–659. 10.1038/ki.2014.296 [DOI] [PubMed] [Google Scholar]
- 11.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 12.Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB, et al. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18:721–726. [PubMed] [Google Scholar]
- 13.Chen HL, Hsiao FY, et al. Risk of hospitalization and healthcare cost associated with Diabetes Complication Severity Index in Taiwan's National Health Insurance Research Database. J Diabetes Complications. 2014;28:612–616. 10.1016/j.jdiacomp.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 14.Davis SN, Mann S, Galassetti P, Neill RA, Tate D, Ertl AC, et al. Effects of Differing Durations of Antecedent Hypoglycemia on Counterregulatory Responses to Subsequent Hypoglycemia in Normal Humans. Diabetes. 2000. November;49(11):1897–903. [DOI] [PubMed] [Google Scholar]
- 15.Yun JS, Ko SH, Ko SH, Song KH, Ahn YB, Yoon KH, et al. Presence of macroalbuminuria predicts severe hypoglycemia in patients with type 2 diabetes: a 10-year follow-up study. Diabetes Care 2013;36:1283–9. 10.2337/dc12-1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quilliam BJ, Simeone JC, Ozbay AB, et al. Risk factors for hypoglycemia- related hospitalization in patients with type 2 diabetes: a nested case-control study. Clin Ther 2011;33:1781–91. 10.1016/j.clinthera.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 17.Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El- Kebbi IM, et al. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med 2001;161:1653–9. [DOI] [PubMed] [Google Scholar]
- 18.Giorda CB, Ozzello A, Gentile S, Aglialoro A, Chiambretti A, Baccetti F, et al. Incidence and risk factors for severe and symptomatic hypoglycemia in type 1 diabetes. Results of the HYPOS-1 study. Acta Diabetol. 2015. October;52(5):845–53. 10.1007/s00592-015-0713-4 [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ, et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. 10.1038/ki.2009.413 [DOI] [PubMed] [Google Scholar]
- 20.Kan WC, Wang JJ, Wang SY, Sun YM, Hung CY, Chu CC, et al. The new comorbidity index for predicting survival in elderly dialysis patients: a long-term population-based study. PLoS One. 2013;8:e68748 10.1371/journal.pone.0068748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CH, Sheu WH, et al. Hypoglycaemic episodes and risk of dementia in diabetes mellitus: 7-year follow-up study. J Intern Med. 2013;273:102–110. 10.1111/joim.12000 [DOI] [PubMed] [Google Scholar]
- 22.ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 23.Thompson AE, et al. JAMA patient page. Hypoglycemia. JAMA. 2015;313:1284 10.1001/jama.2015.0876 [DOI] [PubMed] [Google Scholar]
- 24.Biesenbach G, Raml A, Schmekal B, Eichbauer-Sturm G, et al. Decreased insulin requirement in relation to GFR in nephropathic Type 1 and insulin-treated Type 2 diabetic patients. Diabet Med. 2003;20:642–645. [DOI] [PubMed] [Google Scholar]
- 25.Snyder RW, Berns JS, et al. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17:365–370. 10.1111/j.0894-0959.2004.17346.x [DOI] [PubMed] [Google Scholar]
- 26.Horton ES, Johnson C, Lebovitz HE, et al. Carbohydrate metabolism in uremia. Ann Intern Med. 1968;68:63–74. [DOI] [PubMed] [Google Scholar]
- 27.Pathak RD, Schroeder EB, Seaquist ER, Zeng C, Lafata JE, Thomas A, et al. Severe Hypoglycemia Requiring Medical Intervention in a Large Cohort of Adults With Diabetes Receiving Care in U.S. Integrated Health Care Delivery Systems: 2005–2011. Diabetes Care. 2016;39:363–370. 10.2337/dc15-0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostev K, Dippel FW, Rathmann W, et al. Predictors of hypoglycaemia in insulin-treated type 2 diabetes patients in primary care: a retrospective database analysis. Prim Care Diabetes. 2014;8:127–131. 10.1016/j.pcd.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 29.Gitt AK, Bramlage P, Binz C, Krekler M, Plate T, Deeg E, et al. Hypoglycaemia is more frequent in type 2 diabetic patients with co-morbid vascular disease: an analysis of the DiaRegis registry. Eur J Prev Cardiol. 2012;19:765–772. 10.1177/1741826711411104 [DOI] [PubMed] [Google Scholar]
- 30.Durán-Nah JJ, Rodríguez-Morales A, Smitheram J, Correa-Medina C, et al. Risk factors associated with symptomatic hypoglycemia in type 2 diabetes mellitus patients. Rev Invest Clin. 2008;60:451–458. [PubMed] [Google Scholar]
- 31.Lin YY, Hsu CW, Sheu WH, Chu SJ, Wu CP, Tsai SH, et al. Risk factors for recurrent hypoglycemia in hospitalized diabetic patients admitted for severe hypoglycemia. Yonsei Med J. 2010;51:367–374. 10.3349/ymj.2010.51.3.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raimann JG, Kruse A, Thijssen S, Kuntsevich V, Dabel P, Bachar M, et al. Metabolic effects of dialyzate glucose in chronic hemodialysis: results from a prospective, randomized crossover trial. Nephrol Dial Transplant. 2012;27:1559–1568. 10.1093/ndt/gfr520 [DOI] [PubMed] [Google Scholar]
- 33.Wideröe TE, Smeby LC, Myking OL, et al. Plasma concentrations and transperitoneal transport of native insulin and C-peptide in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 1984;25,82–87. [DOI] [PubMed] [Google Scholar]
- 34.Himmelfarb J1, Ikizler TA, et al. Hemodialysis. N Engl J Med. 2010;363:1833–1845. 10.1056/NEJMra0902710 [DOI] [PubMed] [Google Scholar]
- 35.Burmeister JE, Scapini A, da Rosa Miltersteiner D, da Costa MG, Campos BM, et al. Glucose-added dialysis fluid prevents asymptomatic hypoglycaemia in regular haemodialysis. Nephrol Dial Transplant 2007; 22: 1184–1189. 10.1093/ndt/gfl710 [DOI] [PubMed] [Google Scholar]
- 36.Simic-Ogrizovic S, Backus G, Mayer A, Vienken J, Djukanovic L, Kleophas W, et al. The influence of different glucose concentrations in haemodialysis solutions on metabolism and blood pressure stability in diabetic patients. Int J Artif Organs. 2001. December;24(12):863–9. [PubMed] [Google Scholar]
- 37.Burmeister JE, Campos JF, Miltersteiner DR, et al. Effect of different levels of glucose in the dialysate on the risk of hypoglycemia during hemodialysis in diabetic patients. J Bras Nefrol. 2012. Oct-Dec;34(4):323–7. [DOI] [PubMed] [Google Scholar]
- 38.Lindholm B, Karlander SG, et al. Glucose tolerance in patients undergoing continuous ambulatory peritoneal dialysis. Acta Med Scand 1986;220:477–483. [DOI] [PubMed] [Google Scholar]
- 39.Szeto CC, Chow KM, Kwan BC, Chung KY, Leung CB, Li PK, et al. New-onset hyperglycemia in nondiabetic Chinese patients started on peritoneal dialysis. Am J Kidney Dis 2007;49:524–532. 10.1053/j.ajkd.2007.01.018 [DOI] [PubMed] [Google Scholar]
- 40.Tzamaloukas AH, Murata GH, Eisenberg B, Murphy G, Avasthi PS, et al. Hypoglycemia in diabetics on dialysis with poor glycemic control: hemodialysis versus continuous ambulatory peritoneal dialysis. Int J Artif Organs. 1992. July;15(7):390–2. [PubMed] [Google Scholar]
- 41.Yakubovich N, Gerstein HC, et al. Serious cardiovascular outcomes in diabetes: the role of hypoglycemia. Circulation. 2011;123:342–348. 10.1161/CIRCULATIONAHA.110.948489 [DOI] [PubMed] [Google Scholar]
- 42.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. 10.1056/NEJMoa1003795 [DOI] [PubMed] [Google Scholar]
- 43.Goto A, Arah OA, Goto M, Terauchi Y, Noda M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533 10.1136/bmj.f4533 [DOI] [PubMed] [Google Scholar]
- 44.ORIGIN Trial Investigators, Mellbin LG, Rydén L, Riddle MC, Probstfield J, Rosenstock J, et al. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J. 2013;34(40):3137–3144. 10.1093/eurheartj/eht332 [DOI] [PubMed] [Google Scholar]
- 45.Uttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510–533. 10.1053/j.ajkd.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 46.Allon M, et al. Evidence-based cardiology in hemodialysis patients. J Am Soc Nephrol. 2013;24:1934–1943. 10.1681/ASN.2013060632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the National Health Insurance Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Contact information for the National Health Insurance Research Data Access: Center for Biomedical Resources of NHRI, 35 Keyan Road, Zhunan, Miaoli County 35053, Taiwan. Tel: 037-246166ext.33603 eMail: nhird@nhri.org.tw.