Abstract
Although the number of laparoscopic liver resections (LRRs) has increased, studies of surgical outcomes in comparison with the conventional open approach are limited. The purpose of this study was to analyze the surgical outcomes (safety and efficacy) of LLR versus open liver resection (OLR) for hepatocellular carcinoma (HCC).
We collected data on all patients who received liver resection for HCC between April 2015 and September 2016 in our institution, and retrospectively investigated the demographic and perioperative data, and also surgical outcomes.
Laparoscopic liver resection was performed in 225 patients and OLR in 291. In patients who underwent minor hepatectomy, LLR associated with a shorter duration of operation time (200 vs 220 minutes; P < 0.001), less blood loss (100 vs 225 mL; P < 0.001), lower transfusion rate (3.0% vs 12.0%; P = 0.012), and shorter postoperative hospital stay (6 vs 7 days; P < 0.001) compared with OLR. Dietary recovery was relatively fast in the group of LLR, but there were no significant differences in hepatic inflow occlusion rate, complication rate, and transfusion volume. Patients who received major hepatectomy had a longer duration of operation (240 vs 230 minutes; P < 0.001), less blood loss (200 vs 400 mL; P < 0.001), lower transfusion rate (4.8% vs 16.5%; P = 0.002), lower hepatic inflow occlusion rate (68.3% vs 91.7%; P < 0.001), and shorter postoperative hospital stay (6 vs 8 days; P < 0.001). Complication rate (P = 0.366) and transfusion volume (P = 0.308) did not differ between groups.
Laparoscopic liver resection is a feasible and safe alternative to OLR for HCC when performed by a surgeon experienced with the relevant surgical techniques, associated with less blood loss, lower transfusion rate, a rapid return to a normal diet, and shorter postoperative hospital stay with no compromise in complications. Further, long-term follow-up should be acquired for adequate evaluation for survival.
Keywords: hepatocellular carcinoma, laparoscopic liver resection, surgical outcomes
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver.[1] Liver resection, local ablation, and liver transplantation provide potentially curative therapy for HCC.[2] Laparoscopic liver resection (LLR) was first introduced in the early 1990s,[3] but advancements in laparoscopic approach for liver resection has not been gaining popularity with a fast pace owing to the inherent risks such as lack of tactile sensation, massive bleeding, bile duct injury, and gas embolism.[4] For these reasons, the open liver resection (OLR) has always been the standard approach for liver cancer. The First International Consensus Conference on Laparoscopic Liver Resection (ICCLLR) was held in Louisville in 2008.[4] It defined the minor LLRs should be a standard practice.[5] Since then, with the improvement in technology and equipment, the number of LLRs has increased rapidly worldwide, and extended to major resection,[6,7] robotic hepatectomy,[8,9] anatomical resection,[10] and donor hepatectomy.[11] Moreover, with the improvement in technology and equipment, in the second ICCLLR convened in Morioka in 2014, it was concluded that major LLR is an innovative procedure. It is still in an exploration or learning phase and has incompletely defined risks.[12]
In our institute, with the refinements in laparoscopic instruments and accumulated experience with open liver surgery and laparoscopic surgery for various liver resections, LLR has become a common method of treatment for HCC. Nonetheless, LLR remains challenging because it requires adequate handling of bleeding and important structures. A few recent matched studies demonstrated advantages of LLR over conventional OLR in terms of blood loss, postoperative hospital stays, complications, and return to a normal diet.[13–15] In the present study, we compared the surgical outcomes of patients who had LLR of HCC with that of patients who had OLR in the same period of time for various resection extent.
2. Patients and methods
All procedures described here complied with the Declaration of Helsinki and were approved by the Clinical Trial Ethics Committee of West China Hospital. The ethics committee waived informed consent from patients for the retrospective analysis of existing data because of the low risk of breaching patient confidentiality. We collected patients’, data in a clinical database compiled by West China Hospital, Sichuan University. All consecutive patients who underwent liver resection at our center were identified retrospectively. All liver resections were performed by the same team of experienced hepato-biliary surgeons.
2.1. Patient selection
From April 2015 to September 2016, a total of 534 patients with HCC underwent liver resection in our hospital. Eighteen patients were excluded from the analysis because of open conversion (n = 3), hand-assisted laparoscopic surgery (n = 5), robotic liver resection (n = 1), and underwent combined procedures (n = 9). Thus, 225 patients undergoing LLR and 291 patients undergoing OLR were included for this study. Routine blood tests, viral load, liver function, tumor markers, indocyanine green retention rate at 15 minutes (ICG-R15), aspartate aminotransferase (AST)-to-platelet ratio index (APRI),[16] triphasic computed tomography (CT), or magnetic resonance imaging (MRI) scans were assessed in all patients.
All patients had good preoperative performance status (Eastern Cooperative Oncology Group classes 0 or 1).[17] The indications for LLR were similar to indications for OLR. The criteria for resectability were absence of extrahepatic disseminated disease, en bloc resection suitable and technically feasible, Child-Pugh classification A or B, the remnant liver volume after liver resection was more than 35% of functional liver volume, and no tumor thrombus in the main vein trunk and inferior vena cava. For minor hepatectomy, the cut-off value for ICG-R15 was 20%. An ICG-R15 below 14% was considered favorable for major hepatectomy. The choice between LLR and OLR was dependent on the surgeon's preference. The diagnosis was confirmed after surgery by histopathological examination. The patients undergoing LLR were compared with patients who underwent OLR with respect to preoperative, intraoperative, and postoperative aspects.
2.2. Surgical procedure
For the open approach, the usual wound incision was a right and left (if necessary) subcostal incision with an upward midline incision. After the liver was mobilized, intraoperative ultrasonography was performed routinely to examine the extent of the tumor, define its relationship with the vascular anatomy, and detect whether there were any additional tumors in the liver. Transection of the superficial layer of the liver parenchyma was done by Harmonic scalpel (Ethicon Endo-Surgery, Inc., Cornelia, GA) and electrocautery. Deep parenchymal transection was accomplished with a Cavitron Ultrasonic Surgical Aspirator (CUSA Excel, Valleylab, CO) or the clamp-crushing technique. Low central venous pressure (<5 mm Hg) was used, during liver transection. Hepatic inflow occlusion was performed to control for surgical blood loss in selected patients, with cycles of 15-minute clamp time and 5-minute unclamp time.
Our details of the operation for LLR was described previously.[18,19] The patient was usually placed in a supine position or mild reverse Trendelenburg position, which was determined by the type of operation and the location of the tumor. The primary surgeon stood at the patient's right side or between the patient's legs, and the assistant surgeon and the scopist were positioned on the patient's left. Carbon dioxide pneumoperitoneum was usually achieved by a veress needle and the intra-abdominal pressure was maintained at 13 mm Hg. Five trocars (Ethicon Endo-Surgery Inc., Cincinnati, OH) were usually inserted: three 12-mm ports and two 5-mm ports. Intraoperative ultrasonography was performed similar to the patients receiving OLR. The superficial portion of the liver parenchyma was transected by Harmonic scalpel, whereas the deeper parenchymal transection was accomplished with a combination of CUSA and laparoscopic LigaSure (LigaSure 5-mm BluntTip, Covidien, Boulder, CO). The hepatoduodenal ligament was hanged over using the Pringle maneuver in favorable patients for preventing bleeding during parenchymal transection, with cycles of 15-minute clamp time and 5-minute unclamp time. Small branches of Glisson pedicles and hepatic vein ligated with Hem-o-lock (Weck Closure System, Research Triangle Park, NC) or metal clip. The main hepatic veins and Glisson pedicles were transected by endoscopic linear stapler with 60-mm white cartridge (Endopath Endocutter, EthiconEndo-Surgery Inc.). The resected specimen was placed in a retrieval bag and removed from the abdominal cavity via a suprapubic incision or an upper abdominal midline incision. After irrigation of the surgical bed and hemostasis, fibrin glue was used on the resected liver surface and 1 drainage tube was placed near the surgical bed.
2.3. Definitions and data collection
Patient demographics, and surgical and perioperative data were obtained from the clinical database including duration of surgery, estimated blood loss, length of postoperative hospital stay, complications, and other variables. In OLR, kinds of liver resection have been categorized according to the Brisbane 2000 classification.[20] A minor resection is one in which 2 or fewer Couinaud segments are removed. The definition of major hepatectomy was a resection of 3 or more Couinaud segments. In LLR, resections including posterior superior Couinaud segments 1, 4a, 7, and 8 were considered to be major resections; other hepatectomies were minor resections.[5] Anatomic resection was preferred compared with nonanatomic resection. For comparison of postoperative complications, we used the Clavien–Dindo classification of severity.[21] Liver function was evaluated on the basis of serum total bilirubin, alanine aminotransferase (ALT) levels and albumin, platelet count, and prothrombin time. Postoperative liver dysfunction was defined using the“50-50” criterion[22] on postoperative day 5, with a prothrombin time of less than 50% and a serum total bilirubin level exceeding 50 μmol/L and/or encephalopathy.
2.4. Statistical analysis
The software used to perform these analyses was SPSS statistical software for Windows version 22.0 (SPSS, Inc., Chicago, IL). Continuous variables are expressed as a median with range for skewed distributions or mean ± standard deviation. Differences in continuous variables between groups were carried out using the Student t test, whereas the Mann–Whitney U test was used to compare continuous variables with skewed distributions, and a chi-square or Fisher exact test was used to compare categorical variables. P values <0.05 were considered statistically significant.
3. Results
3.1. Patient demographics and resection types
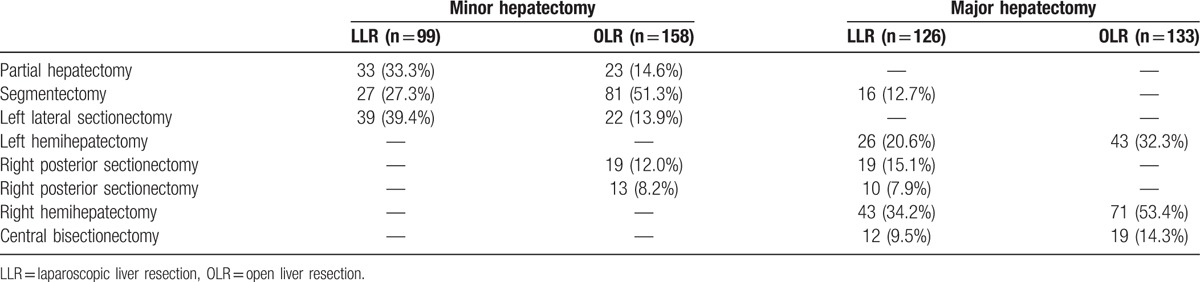
In all, 516 patients who underwent liver resection were enrolled in this study: 225 received LLR and 291 received OLR. Conversion to laparotomy was required in 3 (1.3%) patients because of uncontrolled bleeding, who were excluded from the study. The median patient age was 51 years (range 12–80), and 406 (78.7%) patients were men (Table 1). In the minor resection group, 99 (38.5%) patients underwent LLR, including 33 partial hepatectomy, 27 segmentectomy, and 39 left lateral sectionectomy. One hundred fifty-eight (61.5%) patients underwent OLR, including 23 partial hepatectomy, 81 segmentectomy, 22 left lateral sectionectomy, 19 right posterior sectionectomy, and 13 right anterior sectionectomy. In the major resection group, 126 (48.6%) patients received LLR, including 16 segmentectomy, 26 left hemihepatectomy, 19 right posterior sectionectomy, 10 right anterior sectionectomy, 43 right hemihepatectomy, and 12 central bisectionectomy. One hundred thirty-three (51.4%) patients received OLR, including 43 left hemihepatectomy, 71 right hemihepatectomy, and 19 central bisectionectomy. The detailed description of the type of liver resection can be found in Table 2.
Table 1.
Clinical characteristics of the 2 groups of patients.
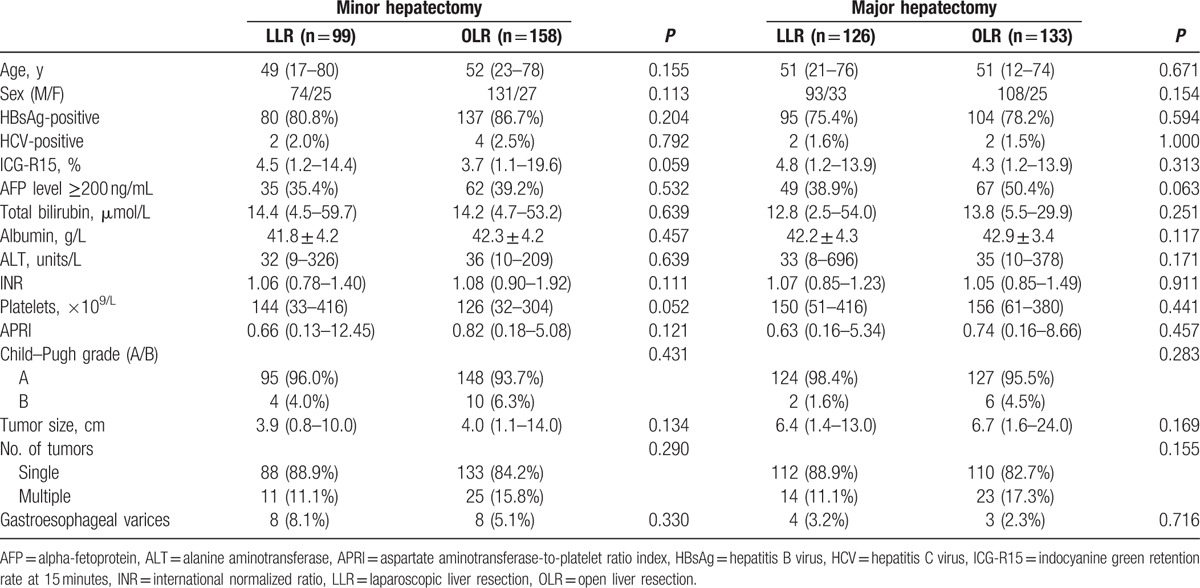
Table 2.
Types of liver resection.
3.2. Baseline characteristics comparison between OLR and LLR group
Patient baseline characteristics in the LLR and OLR groups are summarized in Table 1. Both in minor resection and major resection subgroups, comparing the LLR group and the OLR group, there was no difference in age, sex, hepatitis B infection, or hepatitis C infection. The 2 groups of patients had comparable liver function in terms of serum levels of total bilirubin, albumin, ALT, indocyanine green retention rate, international normalized ratio, and APRI. There were no notable differences in alpha-fetoprotein, tumor size, and platelet count between the 2 groups. The majority of the enrolled patients (494 of 516, 95.7%) were Child–Pugh grade A. Twelve (5.3%) patients of LLR and 11 (3.8%) patients of OLR were diagnosed with gastroesophageal varices. The number of tumor classes single or multiple patients accounted for 443 (85.9%) and 73 (14.1%) individuals, respectively.
3.3. Comparison of the perioperative outcomes
Table 3 summarizes the perioperative outcomes between OLR and LLR group. In the minor resection subgroup, the median duration of surgery was 200 minutes (65–330) in the LLR group and 220 minutes (120–550) in the OLR group (P < 0.001). The median estimated blood loss in LLR was 100 mL (10–1000), whereas that of OLR was 225 mL (50–1600). The transfusion rate was 3 (3.0%) and 19 (12.0%), respectively, and significantly high in case of OLR (P = 0.012), but there were no significant differences in transfusion volume. Hepatic inflow occlusion rates were similar in both groups of patients. The patients started diet significantly later for the OLR than for LLR. The median hospital stay was 6 days (2–16) in the laparoscopic group and 7 days in the open group (3–24) (P < 0.001). Complications occurred in 12 (12.1%) patients in the LLR group and 22 (13.9%) patients in the OLR group (P = 0.678). There were no cases of mortality when they were in hospital. Clavien–Dindo classes I, II, and III patients accounted for 2 (16.7%), 8 (66.6%), and 2 (16.7%) individuals after LLR, respectively. Bleeding requiring operation (IIIb) occurred in 2 cases of OLR. Serum ALT levels were significantly lower in the OLR group than in the LLR group on days 1, 3, and 5 after surgery, whereas there were no significant differences in serum total bilirubin and albumin levels, except albumin on day 1 after operation (Table 4).
Table 3.
Operation details and surgical outcomes in the 2 groups.
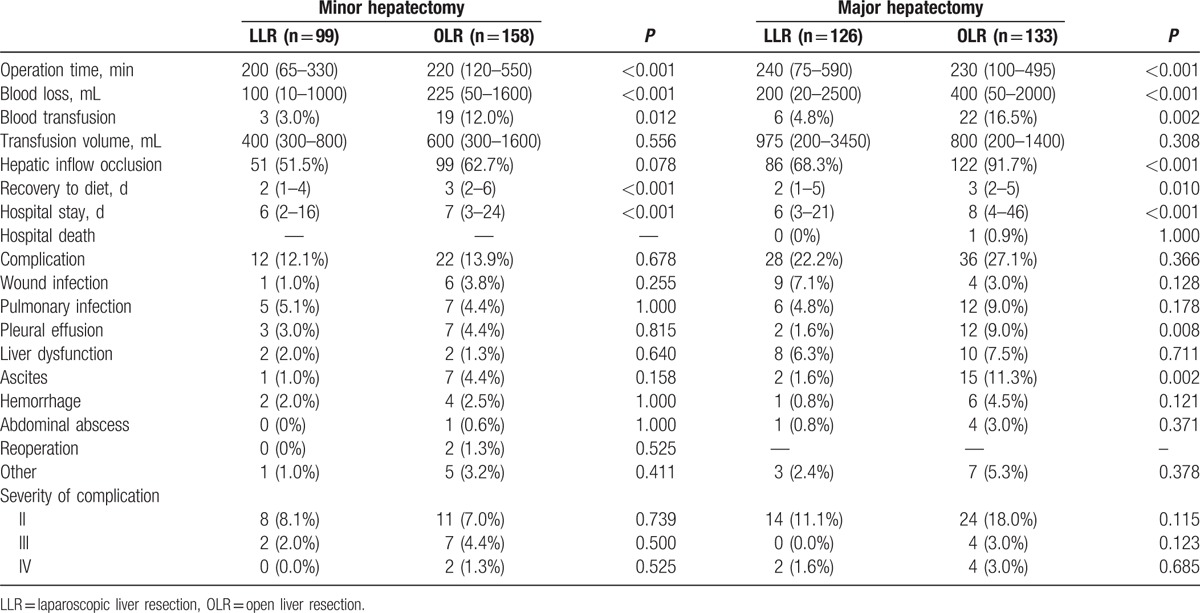
Table 4.
Markers of postoperative liver function.
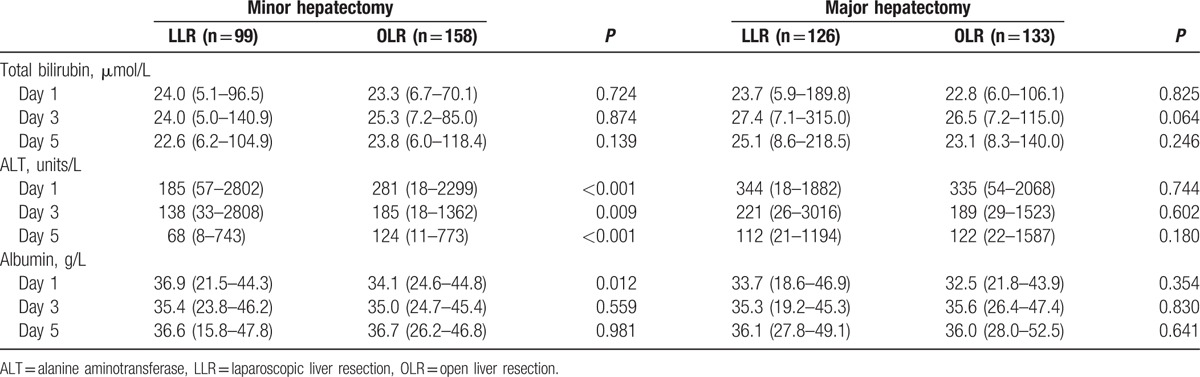
In the major resection subgroup, the duration of operation was longer for LLR than for OLR, whereas the blood loss, transfusion rate, and hepatic inflow occlusion was lower. Postoperatively, the patients started diet significantly earlier in the LLR group: 2 days (1–5) of postoperative fasting in the LLR group and 3 days (2–5) of postoperative fasting in the OLR group (P = 0.010), which consequently resulted in shorter hospitalization: 6 (3–21) days in the LLR group and 8 (4–46) days in the OLR group (P < 0.001). There was 1 death in the open group because of liver failure and sepsis. The morbidity rate was 27.1% in the open group and 22.2% in the laparoscopic group (P = 0.366). There were more patients with pleural effusion in the OLR group than in the LLR group, and more patients with ascites in the OLR group than in the LLR group. Grade II and III complication was 14 and 0 in the LLR group, and 24 and 4 in the OLR group (P = 0.115 and 0.123, respectively). Significant postoperative complications (IV) occurred in 2 cases of LLR and 4 cases of OLR. Serum total bilirubin levels, ALT, and albumin were not significantly different on days 1, 3, and 5 after surgery.
4. Discussion
As shown by an increasing number of reports, LRR is gaining popularity performed for patients with various liver diseases.[23–25] Although the high vascularity of the liver parenchyma makes the operation technically very challenging, there are many advantages of LRR.[14] In our institution, more than 100 liver transplantations, 200 LLRs, and 700 OLRs are done annually. This study shows conclusively that LLR appears to be comparable or superior to OLR regardless of the resection extent. The LLR group had not only a better cosmetic effect, but also had a superior result on surgical outcomes such as total blood loss, blood transfusion rate, recovery to diet, and hospital stay.
In the 2 subgroups, the baseline characteristics of both groups were similar. Consistent with previous studies, the conversion rate to open hepatectomy in our study was 1.3%; in the published literature, general conversion rates ranged from 0% to 20%.[26,27] A comprehensive assessment of preoperative imaging studies, intraoperative ultrasonography, tumor characteristics, and underlying liver condition can help the surgeon prevent conversion to open hepatectomy. Several retrospective studies have demonstrated that the survival rate of patients operated on by anatomical resection was superior to that of patients operated on by nonanatomical resection. If the remnant liver function is tolerable, anatomical liver resection was preferred compared with nonanatomic resection in both LLR and OLR groups.
With regard to surgical outcomes, the LLR group had significantly less blood loss and resulted in lower intraoperative transfusion rate, which is contradictory to many people's impression. This was supported by published literature.[13–15,28,29] These findings could be explained by the following reasons: intraoperative pneumoperitoneum, image magnification enabling more meticulous hemostasis, sophisticated laparoscopic instruments, and minimal invasive wounds. The amount of blood loss is a factor that has a prejudicial impact on both short and long-term outcomes. It has also been reported that perioperative transfusion could lead to poor prognosis.[30,31] A surgeon should always exercise utmost caution during hepatectomy, especially in laparoscopic, to optimize surgical outcomes.[32,33]
Regarding the operation times, the duration of surgery was significantly shorter for LLR than OLR in the minor resection subgroup, but LLR was associated with a increased operation time in the major resection subgroup. There are several explanations for the difference in the duration of surgery between the 2 groups. For the minor resection group, LLR is a standard practice with mature technology and minimal invasive wounds which contribute to operation time. But for the major resection group, LLR is still in an exploration or learning phase, affiliated with technical difficulty such as liver mobilization, vascular control, inability to perform manual palpation, and working with the deeper regions of the liver. The longer duration of surgery might be related to perioperative complications; however, our results were comparable between the 2 groups. The comparative studies reported conflicting results between LLR and OLR: Yoon et al[13] reported lower complications in LLR than in OLR (6.9% vs 22.4%; P = 0.02), whereas Cheung et al[14] reported similar complication rates (6.3% vs 18.8%; P = 0.184). Because the liver-related complications such as liver dysfunction, ascites, and liver failure are much more related to volume of liver resected, background liver characteristics, and hepatic inflow occlusion rates than surgical approach.
The minimal invasive wounds of the abdominal wall may be contributed to the reduction of pain at surgical site and early recover dietary, coupled with LLR was comparable to OLR in terms of postoperative liver function and complications. Therefore, the patients who underwent LLR can early discharge from hospital. In the present study, we provide further evidence for the safety and feasibility of LLR from these aspects. However, there were some limitations of the present study. This study retrospectively analyzed the data regarding patient demographics and surgical outcomes. In addition, the single-center and long-term outcomes were not assessed, which limited the validity of the study. Further multicenter, prospective, and randomized controlled trials should be performed to overcome these limitations.
5. Conclusions
Conclusively, LLR is a feasible and safe alternative to OLR for HCC when performed by a surgeon experienced with the relevant surgical techniques, associated with less blood loss, lower transfusion rate, a rapid return to a normal diet, and shorter postoperative hospital stay with no compromise in complications. Further, long-term follow-up should be acquired for adequate evaluation for survival.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, ALT = alanine aminotransferase, APRI = aspartate aminotransferase-to-platelet ratio index, CT = computed tomography, MRI = magnetic resonance imaging, HCC = hepatocellular carcinoma, ICCLLR = International Consensus Conference on Laparoscopic Liver Resection, ICG-R15 = indocyanine green retention rate at 15 minutes, LLR = laparoscopic liver resection, OLR = open liver resection.
C.J.H. and L.H.Y. contributed equally to this work.
Authors’ contributions: Designed the research—C.J.H., L.B., and W.Y.G. Performed the research—C.J.H., L.H.Y., L.F., L.B., and W.Y.G. Analyzed the data—C.J.H. Wrote the paper—C.J.H. and L.B. Contributed to the operations—C.J.H., L.H.Y., F.L., L.B., and W.Y.G.
The authors have no conflicts of interest or financial ties to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- [3].Reich H, McGlynn F, DeCaprio J, et al. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956–8. [PubMed] [Google Scholar]
- [4].Vibert E, Perniceni T, Levard H, et al. Laparoscopic liver resection. Br J Surg 2006;93:67–72. [DOI] [PubMed] [Google Scholar]
- [5].Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825–30. [DOI] [PubMed] [Google Scholar]
- [6].Lin NC, Nitta H, Wakabayashi G. Laparoscopic major hepatectomy: a systematic literature review and comparison of 3 techniques. Ann Surg 2013;257:205–13. [DOI] [PubMed] [Google Scholar]
- [7].Dagher I, Gayet B, Tzanis D, et al. International experience for laparoscopic major liver resection. J Hepatobiliary Pancreat Sci 2014;21:732–6. [DOI] [PubMed] [Google Scholar]
- [8].Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549–55. [DOI] [PubMed] [Google Scholar]
- [9].Kim JK, Park JS, Han DH, et al. Robotic versus laparoscopic left lateral sectionectomy of liver. Surg Endosc 2016;30:4756–64. [DOI] [PubMed] [Google Scholar]
- [10].Ho CM, Wakabayashi G, Nitta H, et al. Total laparoscopic limited anatomical resection for centrally located hepatocellular carcinoma in cirrhotic liver. Surg Endosc 2013;27:1820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takahara T, Wakabayashi G, Hasegawa Y, et al. Minimally invasive donor hepatectomy: evolution from hybrid to pure laparoscopic techniques. Ann Surg 2015;261:e3–4. [DOI] [PubMed] [Google Scholar]
- [12].Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619–29. [DOI] [PubMed] [Google Scholar]
- [13].Yoon SY, Kim KH, Jung DH, et al. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc 2015;29:2628–34. [DOI] [PubMed] [Google Scholar]
- [14].Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 2013;257:506–11. [DOI] [PubMed] [Google Scholar]
- [15].Takahara T, Wakabayashi G, Konno H, et al. Comparison of laparoscopic major hepatectomy with propensity score matched open cases from the National Clinical Database in Japan. J Hepatobiliary Pancreat Sci 2016;23:721–34. [DOI] [PubMed] [Google Scholar]
- [16].Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology 2007;46:912–21. [DOI] [PubMed] [Google Scholar]
- [17].Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- [18].Li H, Wei Y, Li B, et al. The first case of total laparoscopic living donor right hemihepatectomy in mainland China and literature review. Surg Laparosc Endosc Percutan Tech 2016;26:172–5. [DOI] [PubMed] [Google Scholar]
- [19].Liu F, Zhang J, Lei C, et al. Feasibility of laparoscopic major hepatectomy for hepatic paragonimiasis: two case reports. Medicine (Baltimore) 2016;95:e4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351–5. [DOI] [PubMed] [Google Scholar]
- [21].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 2000;62:299–307. [DOI] [PubMed] [Google Scholar]
- [23].Reddy SK, Tsung A, Geller DA. Laparoscopic liver resection. World J Surg 2011;35:1478–86. [DOI] [PubMed] [Google Scholar]
- [24].Calise F, Giuliani A, Sodano L, et al. Segmentectomy: is minimally invasive surgery going to change a liver dogma. Updates Surg 2015;67:111–5. [DOI] [PubMed] [Google Scholar]
- [25].Kang SH, Kim KH, Shin MH, et al. Surgical outcomes following laparoscopic major hepatectomy for various liver diseases. Medicine (Baltimore) 2016;95:e5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Polignano FM, Quyn AJ, de Figueiredo RS, et al. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc 2008;22:2564–70. [DOI] [PubMed] [Google Scholar]
- [27].Topal B, Fieuws S, Aerts R, et al. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc 2008;22:2208–13. [DOI] [PubMed] [Google Scholar]
- [28].Yin Z, Fan X, Ye H, et al. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol 2013;20:1203–15. [DOI] [PubMed] [Google Scholar]
- [29].Nguyen KT, Marsh JW, Tsung A, et al. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348–56. [DOI] [PubMed] [Google Scholar]
- [30].Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397–406. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860–9. [discussion 869–870]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wei AC, Tung-Ping PR, Fan ST, et al. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg 2003;90:33–41. [DOI] [PubMed] [Google Scholar]
- [33].Giuliani A, Aldrighetti L, Di BF, et al. Total abdominal approach for postero-superior segments (7, 8) in laparoscopic liver surgery: a multicentric experience. Updates Surg 2015;67:169–75. [DOI] [PubMed] [Google Scholar]


