Abstract
The antioxidant properties of tryptophan and some of its oxidative metabolites were examined by measuring how efficiently they inhibited peroxyl radical-mediated oxidation of phosphatidylcholine liposomes and B-phycoerythrin. Low micromolar concentrations of 5-hydroxytryptophan, 3-hydroxykynurenine, xanthurenic acid, or 3-hydroxyanthranilic acid, but not their corresponding nonhydroxylated metabolic precursors, scavenged peroxyl radicals with high efficiency. In particular, 3-hydroxykynurenine and 3-hydroxyanthranilic acid protected B-phycoerythrin from peroxyl radical-mediated oxidative damage more effectively than equimolar amounts of either ascorbate or Trolox (a water-soluble analog of vitamin E). Enzyme activities involved or related to oxidative tryptophan metabolism, as well as endogenous concentrations of tryptophan and its metabolites, were determined within tissues of mice suffering from acute viral pneumonia. Infection resulted in a 100-fold induction of pulmonary indoleamine 2,3-dioxygenase (EC 1.13.11.17) as reported [Yoshida, R., Urade, Y., Tokuda, M. & Hayaishi, O. (1979) Proc. Natl. Acad. Sci. USA 76, 4084-4086]. This was accompanied by a 16- and 3-fold increase in the levels of lung kynurenine and 3-hydroxykynurenine, respectively. In contrast, endogenous concentrations of tryptophan and xanthurenic acid did not increase and 3-hydroxyanthranilic acid could not be detected. The activity of the superoxide anion (O2-.)-producing enzyme xanthine oxidase increased 3.5-fold during infection while that of the O2-.-removing superoxide dismutase decreased to 50% of control levels. These results plus the known requirement of indoleamine 2,3-dioxygenase for superoxide anion for catalytic activity suggest that viral pneumonia is accompanied by oxidative stress and that induction of indoleamine 2,3-dioxygenase may represent a local antioxidant defence against this and possibly other types of inflammatory diseases.
Full text
PDF
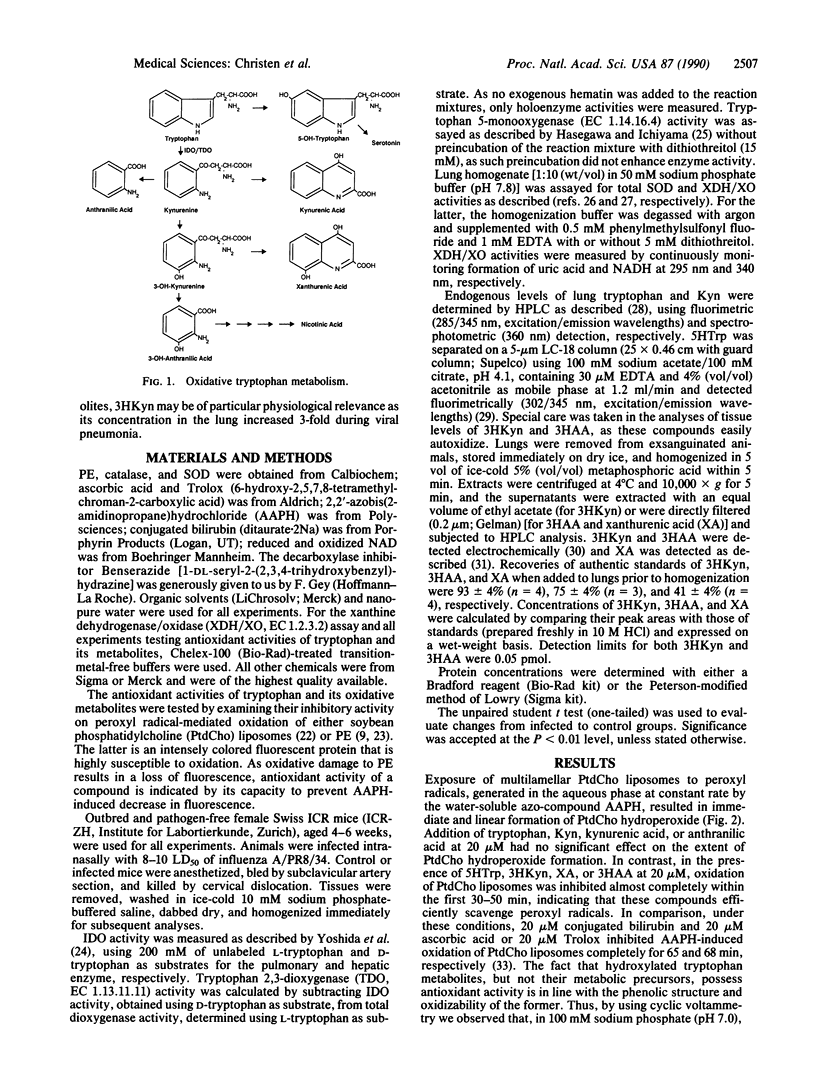
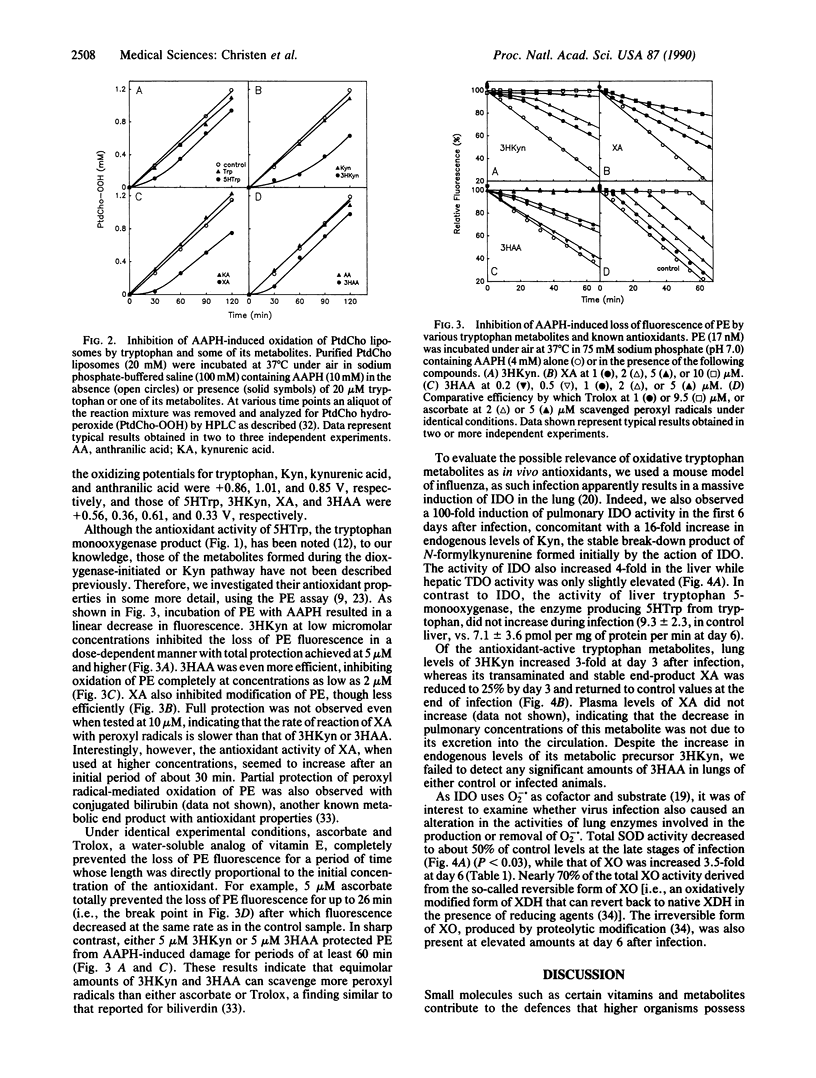
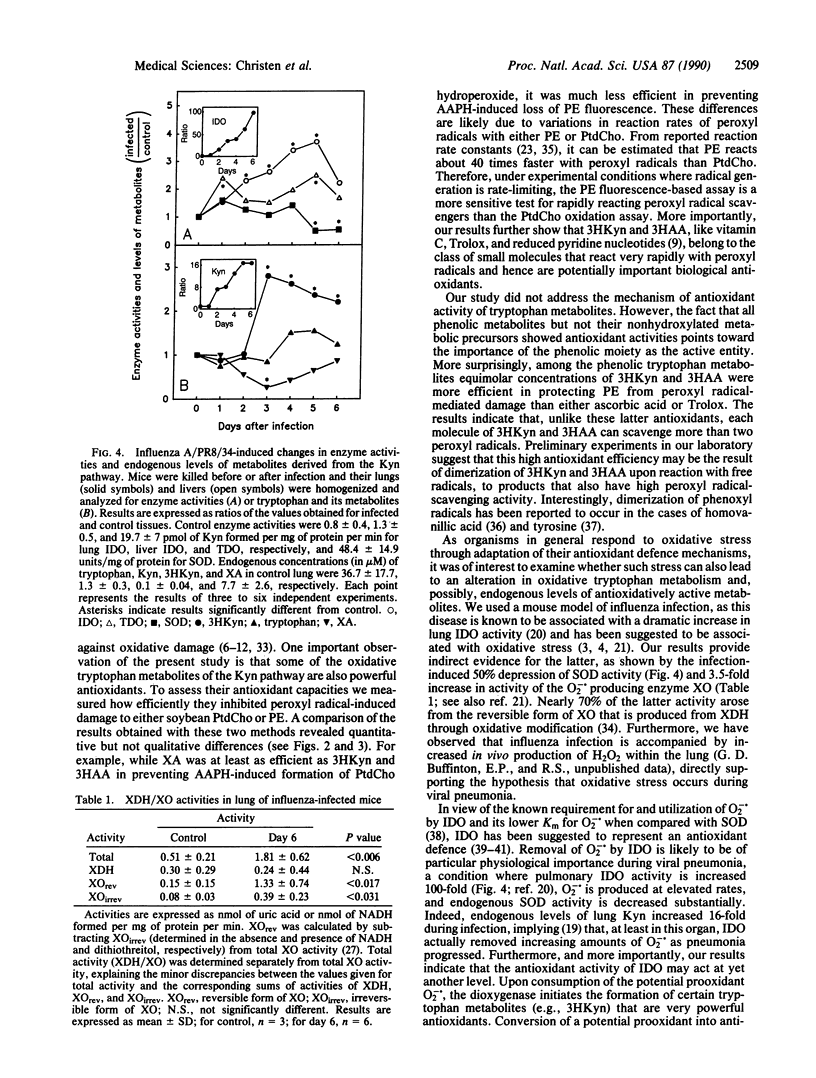

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M., Bertini R., Ghezzi P. Induction of indoleamine dioxygenase by interferon in mice: a study with different recombinant interferons and various cytokines. Biochem Biophys Res Commun. 1988 Apr 15;152(1):237–242. doi: 10.1016/s0006-291x(88)80705-8. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Simic M. G., Sies H. Antioxidant activity of 5-hydroxytryptophan, 5-hydroxyindole, and DOPA against microsomal lipid peroxidation and its dependence on vitamin E. Free Radic Res Commun. 1989;6(1):11–17. doi: 10.3109/10715768909073423. [DOI] [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Sondel P. M., Byrne G. I. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987 Oct 1;139(7):2414–2418. [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Sondel P. M., Byrne G. I. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol. 1989 Jan;45(1):29–34. doi: 10.1002/jlb.45.1.29. [DOI] [PubMed] [Google Scholar]
- Carlin J. M., Ozaki Y., Byrne G. I., Brown R. R., Borden E. C. Interferons and indoleamine 2,3-dioxygenase: role in antimicrobial and antitumor effects. Experientia. 1989 Jun 15;45(6):535–541. doi: 10.1007/BF01990503. [DOI] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley-Yates P. T., Powell A. P., Smith L. L. Pulmonary indoleamine 2,3-dioxygenase activity and its significance in the response of rats, mice, and rabbits to oxidative stress. Toxicol Appl Pharmacol. 1988 Nov;96(2):222–232. doi: 10.1016/0041-008x(88)90082-8. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Glazer A. N. Phycoerythrin fluorescence-based assay for peroxy radicals: a screen for biologically relevant protective agents. Anal Biochem. 1989 Mar;177(2):300–306. doi: 10.1016/0003-2697(89)90056-0. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P., Saccardo B., Bianchi M. Induction of xanthine oxidase and heme oxygenase and depression of liver drug metabolism by interferon: a study with different recombinant interferons. J Interferon Res. 1986 Jun;6(3):251–256. doi: 10.1089/jir.1986.6.251. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Fluorescence-based assay for reactive oxygen species: a protective role for creatinine. FASEB J. 1988 Jun;2(9):2487–2491. doi: 10.1096/fasebj.2.9.3371593. [DOI] [PubMed] [Google Scholar]
- Groseclose E. E., Frank L. The activity of pulmonary indoleamine 2,3-dioxygenase in rats and mice is not altered by oxygen exposure. Biochim Biophys Acta. 1982 Aug 10;705(3):341–347. doi: 10.1016/0167-4838(82)90256-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Hasegawa H., Ichiyama A. Tryptophan 5-monooxygenase from mouse mastocytoma: high-performance liquid chromatography assay. Methods Enzymol. 1987;142:88–92. doi: 10.1016/s0076-6879(87)42013-2. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Hirata F., Ohnishi T., Henry J. P., Rosenthal I., Katoh A. Indoleamine 2,3-dioxygenase: incorporation of 18O2-- and 18O2 into the reaction products. J Biol Chem. 1977 May 25;252(10):3548–3550. [PubMed] [Google Scholar]
- Hunter E. P., Desrosiers M. F., Simic M. G. The effect of oxygen, antioxidants, and superoxide radical on tyrosine phenoxyl radical dimerization. Free Radic Biol Med. 1989;6(6):581–585. doi: 10.1016/0891-5849(89)90064-6. [DOI] [PubMed] [Google Scholar]
- McKelvey T. G., Höllwarth M. E., Granger D. N., Engerson T. D., Landler U., Jones H. P. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol. 1988 May;254(5 Pt 1):G753–G760. doi: 10.1152/ajpgi.1988.254.5.G753. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Szuro-Sudol A., Wellner D., Oca M. J., Granger A. M., Libby D. M., Rothermel C. D., Rubin B. Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989 Mar;57(3):845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito J., Ishiguro I., Murazumi T., Morimoto M. Determination of kynurenine in serum by high-performance liquid chromatography after enzymatic conversion to 3-hydroxykynurenine. Anal Biochem. 1987 Feb 15;161(1):16–19. doi: 10.1016/0003-2697(87)90644-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley L. W., Spitz D. R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984;105:457–464. doi: 10.1016/s0076-6879(84)05064-3. [DOI] [PubMed] [Google Scholar]
- Oda T., Akaike T., Hamamoto T., Suzuki F., Hirano T., Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989 May 26;244(4907):974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Hirata F., Hayaish O. Indoleamine 2,3-dioxygenase. Potassium superoxide as substrate. J Biol Chem. 1977 Jul 10;252(13):4643–4647. [PubMed] [Google Scholar]
- Ozaki Y., Edelstein M. P., Duch D. S. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon gamma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1242–1246. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki Y., Reinhard J. F., Jr, Nichol C. A. Cofactor activity of dihydroflavin mononucleotide and tetrahydrobiopterin for murine epididymal indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1106–1111. doi: 10.1016/0006-291x(86)90339-6. [DOI] [PubMed] [Google Scholar]
- Peterhans E. Chemiluminescence: an early event in the interaction of Sendai and influenza viruses with mouse spleen cells. I. The role of the envelope glycoproteins in the stimulation of chemiluminescence. Virology. 1980 Sep;105(2):445–455. doi: 10.1016/0042-6822(80)90045-8. [DOI] [PubMed] [Google Scholar]
- Peterhans E., Grob M., Bürge T., Zanoni R. Virus-induced formation of reactive oxygen intermediates in phagocytic cells. Free Radic Res Commun. 1987;3(1-5):39–46. doi: 10.3109/10715768709069768. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Okatani Y., Yamanaka S., Kiriyama T. Determination of plasma 5-hydroxytryptophan, 5-hydroxytryptamine, 5-hydroxyindoleacetic acid, tryptophan and melatonin by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1988 Sep 23;431(1):170–176. doi: 10.1016/s0378-4347(00)83081-9. [DOI] [PubMed] [Google Scholar]
- Stocker R., Ames B. N. Potential role of conjugated bilirubin and copper in the metabolism of lipid peroxides in bile. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8130–8134. doi: 10.1073/pnas.84.22.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Peterhans E. Synergistic interaction between vitamin E and the bile pigments bilirubin and biliverdin. Biochim Biophys Acta. 1989 Apr 3;1002(2):238–244. doi: 10.1016/0005-2760(89)90293-2. [DOI] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Sugino K., Dohi K., Yamada K., Kawasaki T. Changes in the levels of endogenous antioxidants in the liver of mice with experimental endotoxemia and the protective effects of the antioxidants. Surgery. 1989 Feb;105(2 Pt 1):200–206. [PubMed] [Google Scholar]
- Urade Y., Yoshida R., Kitamura H., Hayaishi O. Induction of indoleamine 2,3-dioxygenase in alveolar interstitial cells of mouse lung by bacterial lipopolysaccharide. J Biol Chem. 1983 May 25;258(10):6621–6627. [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Werner E. R., Hirsch-Kauffmann M., Fuchs D., Hausen A., Reibnegger G., Schweiger M., Wachter H. Interferon-gamma-induced degradation of tryptophan by human cells in vitro. Biol Chem Hoppe Seyler. 1987 Oct;368(10):1407–1412. doi: 10.1515/bchm3.1987.368.2.1407. [DOI] [PubMed] [Google Scholar]
- Williams S. A., Monti J. A., Boots L. R., Cornwell P. E. Quantitation of xanthurenic acid in rabbit serum using high performance liquid chromatography. Am J Clin Nutr. 1984 Jul;40(1):159–167. doi: 10.1093/ajcn/40.1.159. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Park S. W., Yasui H., Takikawa O. Tryptophan degradation in transplanted tumor cells undergoing rejection. J Immunol. 1988 Oct 15;141(8):2819–2823. [PubMed] [Google Scholar]
- Yoshida R., Urade Y., Nakata K., Watanabe Y., Hayaishi O. Specific induction of indoleamine 2,3-dioxygenase by bacterial lipopolysaccharide in the mouse lung. Arch Biochem Biophys. 1981 Dec;212(2):629–637. doi: 10.1016/0003-9861(81)90406-9. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Urade Y., Tokuda M., Hayaishi O. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4084–4086. doi: 10.1073/pnas.76.8.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]


