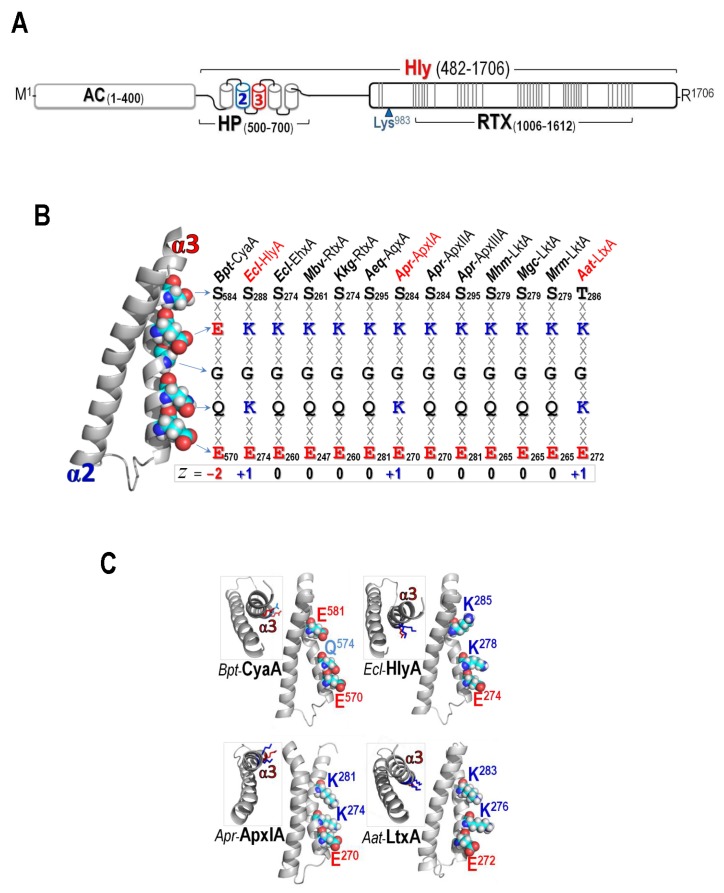
Figure 1.
(A) (Above) Schematic representation of CyaA showing adenylate cyclase (AC) domain and hemolysin (Hly) domain which contains the hydrophobic (HP) region, palmitoylation site (Lys983) and the RTX region. Five putative helices in the HP region are indicated by cylinders where α2 and α3, putative membrane-inserting constituents were marked in blue and red, respectively. Each line in the RTX region represents each repeat of (X-U-X-Gly-Gly-X-Gly-X-Asp, X for any amino acid and U for large hydrophobic residues); (B) Left panel: The homology-based model of the CyaA-Hly α2–α3 hairpin. Side chains at pore-lining region of α3 are shown as van der Waals (vdW) spheres and colored according to atoms: cyan, white, red, and blue for C, H, O, and N, respectively. Right panel: Conserved amino acids at pore-lining regions of thirteen related RTX cytolysins, as mentioned earlier in [25]. The side-chains are highlighted in bold letter and the charged residues are colored in red for negative and blue for positive; and (C) Side view of the hairpins of CyaA-Hly and three highly-active RTX cytolysins, i.e., HlyA from E. coli, ApxIA from Actinobacillus pleuropneumoniae and LtxA from Aggregatibacter actinomycetemcomitans, showing three key side chains at Glu570/Gln574/Glu581 (for CyaA-Hly) and Glu/Lys/Lys (for HlyA, ApxIA, and LtxA) patches. Inset: Top view of individual hairpins. Amino acids are colored according to their charged/polar properties (red is negatively-charged, blue is positively-charged, and light-blue is N-containing polar uncharged) with H atoms omitted.