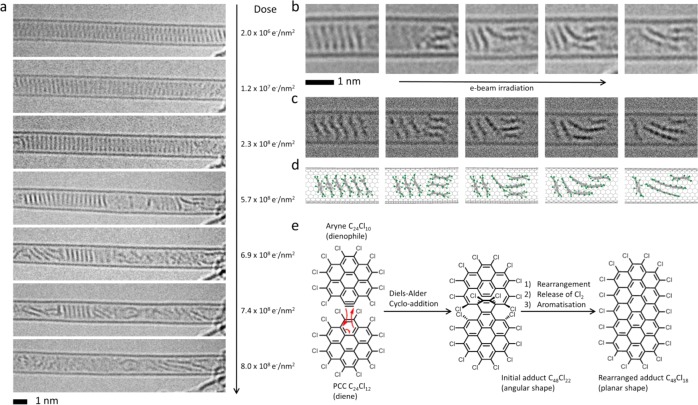
Figure 2.
(a) Time-series AC-HRTEM images showing the transformation of a stack of PCC within a SWNT under 80 keV e-beam exposure (the electron dose accumulated by the molecules during the time series is shown on the right side of each micrograph). (b–d) Close examination of the experimental images (b) and comparison with simulated TEM images (c), generated by exposing the corresponding structural models (d) to limited doses of 1 × 106 e–/nm2 of 80 keV electrons, indicate that intermolecular reactions are possible only when a PCC molecule can change its orientations with respect to the neighboring molecules: Two nonparallel molecules are able to join together to form an angular adduct which gradually transforms into a planar species approximately twice the length of the original PCC. (e) The intermolecular addition reaction observed by AC-HRTEM in carbon nanotubes is consistent with the Diels–Alder cycloaddition of an aryne (molecular formula = C24Cl10) to PCC predicted by DFT calculation and rearrangement of the initial adduct (angular) to an elongated flat polyaromatic molecule, C48Cl18 (planar), which continues reacting with further aryne species leading to the formation of ribbon-like structures terminated with chlorine atoms around the edge.