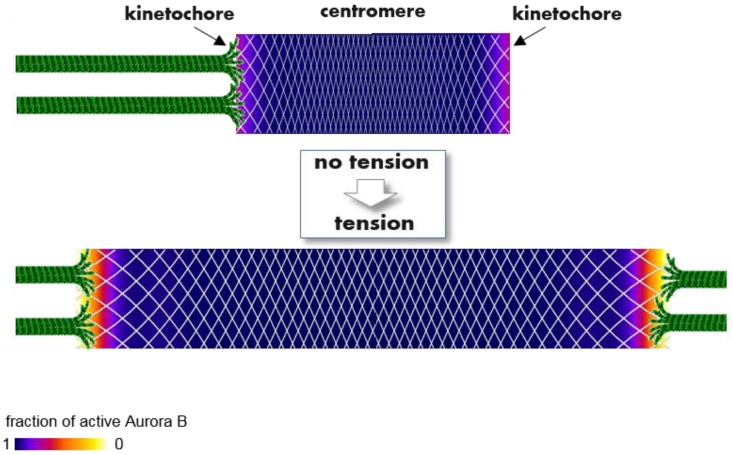
Figure 6.
A molecular model to explain how tension regulates phosphorylation of kinetochore proteins that bind KMTs and regulate their attachment lifetime. Color-coded plots show the spatial distribution of active Aurora B kinase within a continuous flexible matrix (white mesh), encompassing the centromeric chromatin and two sister kinetochores (based on the theoretical model in [89]). Aurora B kinase is enriched strongly in the middle of the centromere (not shown), where it becomes highly active (purple and blue colors) due to trans-molecular auto-phosphorylation. With no tension this activity propagates from the centromere throughout the entire matrix, as active kinase “ignites” the nearby kinase, overcoming opposing phosphatases. As a result, kinase activity at the kinetochores is high with no tension, reducing KMT lifetime. When amphitelic KMTs stretch the connecting matrix, local Aurora B concentration is reduced everywhere (shown by increased spacing of the white mesh). However, the local concentration of active kinase does not decrease proportionally owing to the highly nonlinear, bistable nature of the underlying kinase-phosphatase switch. Aurora B kinase activity remains high within centromeric heterochromatin but drops sharply at the outer kinetochore (orange/red colors), forming phosphorylation gradients within the kinetochores. While this model provides a biophysical explanation for tension-dependent, long-range regulation of Aurora B kinase activity, the physiological significance of the kinetochore activity gradient seen in mammalian cells remains to be understood.