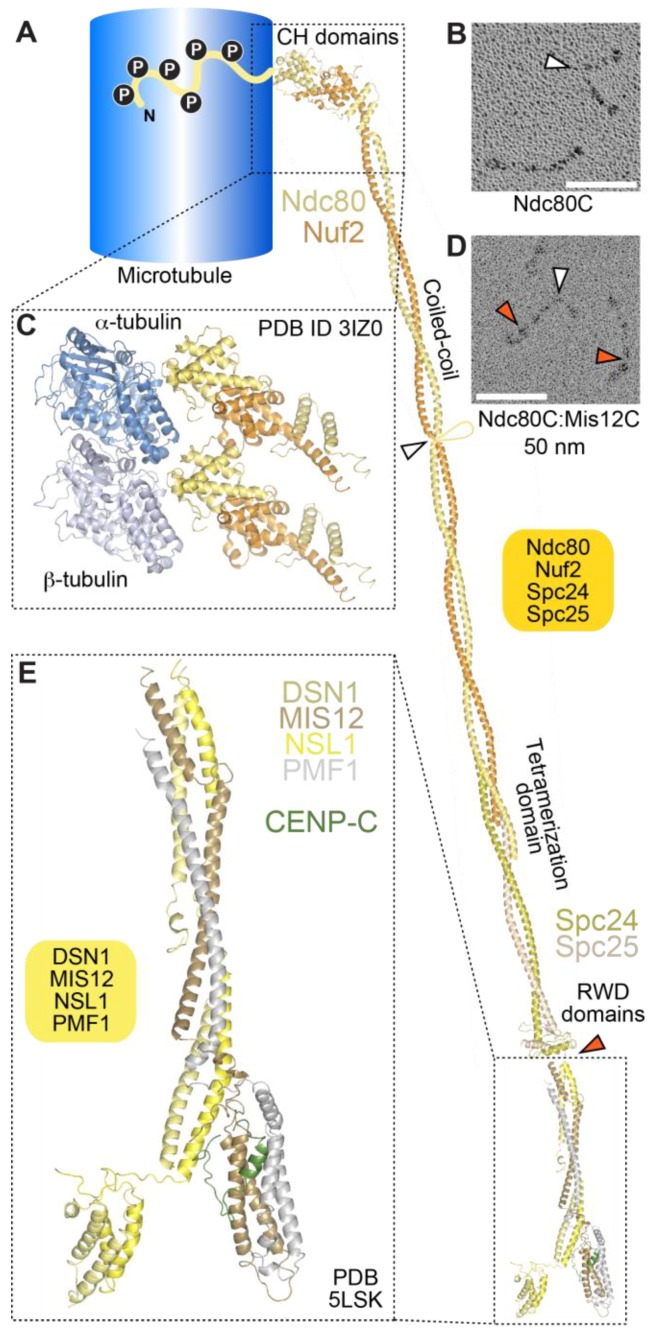
Figure 4.
The NDC80 and MIS12 complexes of the KMN network. (A) The NDC80 complex is highly elongated and interacts with microtubules via calponin-homology (CH) domains in the N-terminal regions of NDC80 and NUF2. A basic N-terminal tail preceding the NDC80 CH domain (depicted as unstructured) is subject to Aurora kinase phosphorylation (Ps in black circles) and regulates microtubule binding. A long coiled-coil, interrupted by a loop (white arrowhead) terminates in a tetramerization domain with SPC24 and SPC25. The latter start with coiled-coils and terminate with RWD domains (red arrowhead), which interact with the MIS12 complex; (B) Rotary shadowing electron microscopy of the NDC80 complex, showing its characteristic dumbbell shape, and an overall length of ~65 nm. Images in (B,D) courtesy of Dr. Pim Huis in ‘t Veld, Max Planck Institute of Molecular Physiology, Dortmund (Germany) [239]; (C) Model from cryo-EM studies of the Ndc80Bonsai complex bound to the microtubule lattice. Only a single α-tubulin:β-tubulin dimer is shown, with two Ndc80Bonsai complexes bound via the toe region; (D) Complexes of the NDC80C and MIS12C are ~85 nm in length; (E) Structural organization of the MIS12 complex bound to the N-terminal region of CENP-C [241]. All structures shown are for the human complexes.