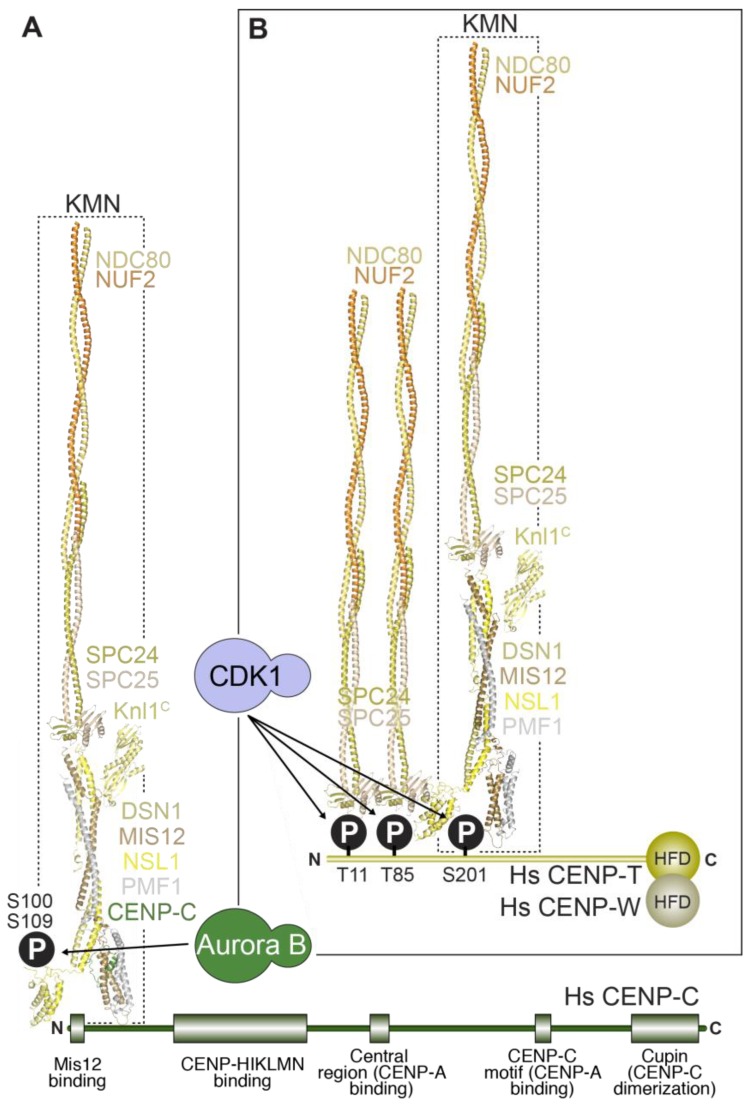
Figure 6.
Linkages between the inner and outer kinetochore. Structures of a portion of the NDC80 complex (the N-terminal globular domains of NDC80 and NUF2 are not shown), the MIS12 complex and the C-terminal kinetochore-targeting domain of KNL1 are used to depict a KMN particle in humans. (A) The first linkage is formed by the interaction of a KMN particle with the N-terminal region of CENP-C. This interaction is enhanced by Aurora B phosphorylation of residues (S100 and S109) in the N-terminal region of the DSN1 subunit of the MIS12 complex; (B) The second linkage involves the interaction of up to two NDC80 complexes with two CDK1-phosphorylated residues (T11 & T85) in the N-terminal region of CENP-T, as well as of a second entire KMN recruited via a CDK1-dependent interaction of the MIS12 complex with S201 of CENP-T [239]. In vitro, CENP-C and CENP-T bind to the MIS12 complex within the KMN network competitively, implying that they cannot be bound to the same KMN [239]. All structures shown are for the human complexes.