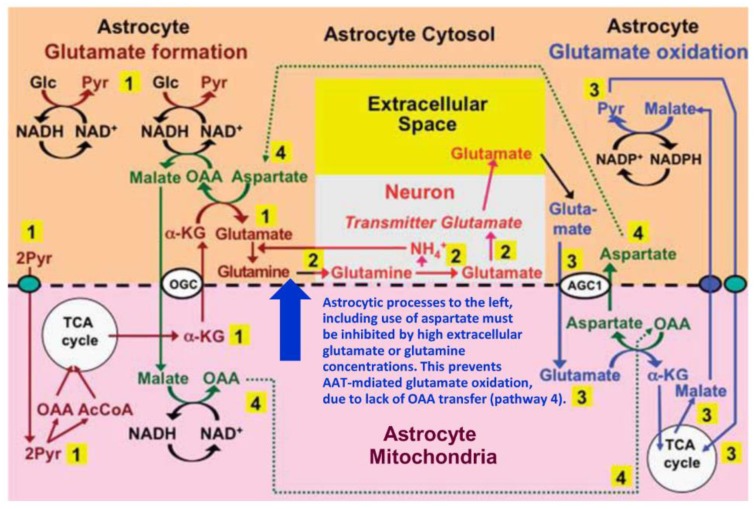
Figure 3.
Proposed pathway for coupled production and metabolism of transmitter glutamate using aspartate transamination for exchange between α-KG and glutamate. Joint pyruvate carboxylase and pyruvate dehydrogenase activation generates a “new” molecule of citrate (lower left corner) as detailed in Figure 1. Citrate-derived α-KG exiting the mitochondrial membrane leaves the astrocytic TCA cycle and is transaminated with aspartate to form glutamate, with concomitant oxaloacetate (OAA) formation from aspartate. The mitochondrial exit of α-KG occurs via the α-ketoglutarate/malate exchanger, generally acknowledged to be expressed in astrocytes, and the cytosolic malate with which it is exchanged, is generated via NADH-supported reduction of oxaloacetate generated from aspartate. Glutamate is amidated to glutamine (pathway 1), which is transferred to glutamatergic neurons (without indication of any extracellular space in the Figure). High extracellular concentrations of glutamate or glutamine (blue arrow) will at least in cultured astrocytes make the astrocytic production of glutamate and glutamine unnecessary (and/or prevent the reaction for thermodynamic reasons) and thereby inhibit glutamate formation from α-KG and the associated transamination of aspartate. In neurons glutamine is in a complex pathway converted to glutamate, accumulated in vesicles and released as transmitter glutamate (pathway 2). Subsequent reuptake of glutamate and oxidative metabolism in astrocytes (pathway 3) is normally of similar magnitude as the production of glutamate described above. Cytosolic glutamate is transferred to mitochondria via the aspartate-glutamate exchanger AGC1 (aralar) in exchange with mitochondrial aspartate generated from OAA formed during the synthesis of glutamate (pathway 4) and re-converted to aspartate during transamination of glutamate to α-KG. In turn, the cytosolic aspartate is used during glutamate synthesis in the transamination of α-KG to glutamate in pathway 1 after transfer via pathway 4. However, when glutamate production from α-KG is inhibited by high extracellular concentrations of glutamate or glutamine (blue arrow) or by excess ammonia (inhibiting the neuronal glutaminase [98]), the exit of aspartate to the cytosol in exchange with glutamate during glutamate oxidation will no longer be compensated for by aspartate use during glutamate formation. This abolishes glutamate oxidation via AAT, so that formation of α-KG must be catalyzed by glutamate dehydrogenase (GDH). This is unlikely normally to take place in vivo since elevated extracellular glutamate only occurs during bursts of neuronal activity and glutamate uptake and glutamine synthesis rapidly clear extra- and intracellular glutamate. Metabolism of α-KG is only shown via malate exit and pyruvate formation. Since this is an oxidation in the cytosol it must be followed by malate aspartate shuttle (MAS)-mediated transfer of a reducing equivalent to the mitochondria. Biosynthesis of glutamine is shown in brown and metabolic degradation of glutamate in blue. Synthesis of glutamine and export from the glia is critical since it restores nitrogen balance (otherwise concentrations of glutamate and aspartate would continuously rise). Redox shuttling and astrocytic release of glutamine and uptake of glutamate are shown in black, and neuronal uptake of glutamine, hydrolysis to glutamate, and its release is shown in red. Reactions involving or resulting from transamination between aspartate and oxaloacetate (OAA) are shown in green. Small blue oval shows pyruvate carrier into mitochondria and small purple oval malate carrier out from mitochondria. AGC1, aspartate/glutamate exchanger, aralar; α-KG, α-ketoglutarate; Glc, glucose; Pyr, pyruvate; OGC, malate/α-ketoglutarate exchanger. AGC1 is for graphical reasons only indicated during the initial part of glutamate oxidation, where it constitutes part of the suggested pathway, but not where it is generally acknowledged to function in MAS, i.e., during synthesis of pyruvate from glucose (pathway 1) and from malate (pathway 3). The glutamine–(GABA) cycle is accordingly extremely dependent upon the abundant expression of AGC1 in astrocytes described above. However, if malate generated during glutamate degradation does not exit the TCA cycle but is further metabolized to α-KG, allowing re-synthesis of another molecule of glutamate from only one molecule of pyruvate and abrogating pyruvate formation from malate (Figure 1) trafficking via AGC1 will be considerably reduced, although certainly not abolished. Slightly modified from [15].