Fig. 5.
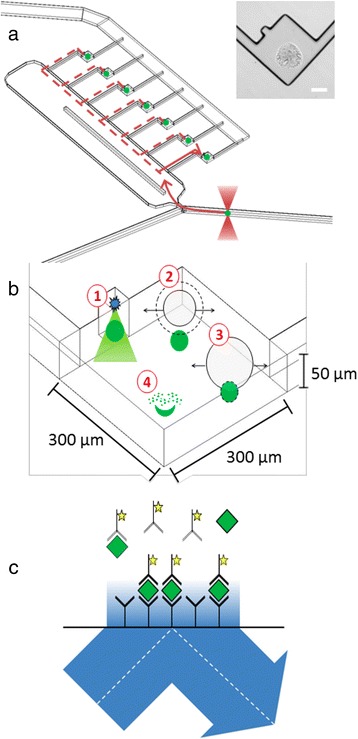
Microfluidic approach to single-cell phosphoproteomics. a The optical trap is used to move cells (green circles) from flow to analysis chambers. Inset: bright-field image of an antibody spot aligned within a chamber. Scale bar = 100 μm. b Single cells (green circles) are lysed by the delivery of a single 6 ns pulse at λ = 1064 nm 10 μm above the center of the cell. (1) At sufficient irradiance the medium breaks down to form a localized plasma; (2) An outwardly propagating shockwave and an expanding cavitation bubble are produced; (3) the cell is lysed due to shear stress from the expanding cavitation bubble; and (4) cellular constituents are released into the chamber. c Single cell protein levels are measured using an antibody spot. Chamber volume is 4.6 nL and results in favorable kinetics. By employing TIRF, only 10 fluorophores within 200 nm of the surface are imaged, which are assumed to be antibody/antigen bound. Reproduced from [129] with permission from the Royal Society of Chemistry © 2011
