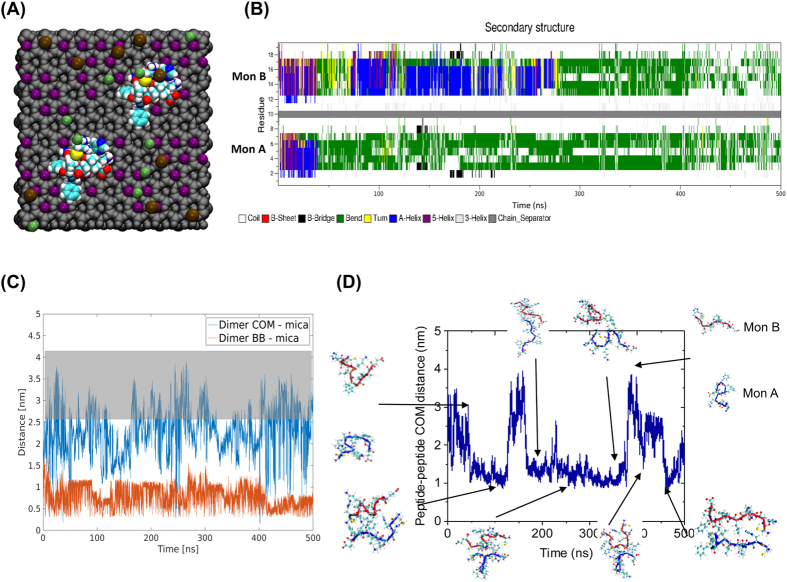
Figure 4. Molecular dynamics simulations of on-surface aggregation of Aβ (14-23) dimers.
(A) Schematic of the simulation system showing van der Waal representation of the atoms. Grey color is the mica structure excluding potassium cations, K+ are purple, Cl− are green, Na+ are brown, while peptides are colored using atomic names in VMD. (B) Time-dependent change of the secondary structure of the peptides determined using DSSP. Solid gray bar separates the two monomer, with monomer A being below the separator. (C) COM distance between dimer and mica surface, blue, and the minimum distance of dimer backbone and mica surface, red, for Mica 1 system as determined by g_mindist. Highlight indicates distance at which dimer is dissociated from mica surface (D) The plot shows COM distance between the two Aβ(14-23) peptides in the Mica 1 system; key events of the simulation are highlighted with a cartoon representation of the dimer, blue represents monomer A and red monomer B.