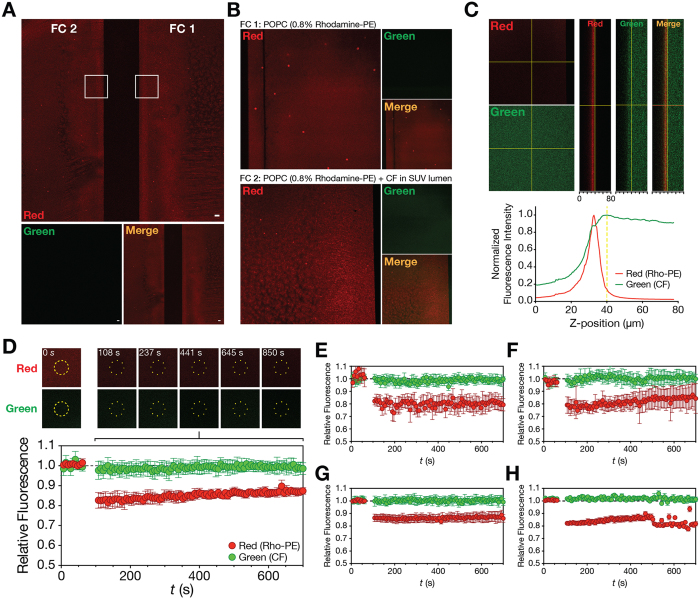
Figure 1. Characterization of the lipid surface formed on a L1 sensor chip.
(A) Confocal images of flow cell (FC) 1 and 2 formed on the L1 sensor chip surface (XY plane) using 10x magnification objectives. POPC SUV labelled with either Rho-PE (control) or Rho-PE and CF were deposited on FC1 and 2, respectively. A 20 μm white scale bar is included. (B) Individual confocal images of FC1 (top) and 2 (bottom) collected at the respective white square ROI in A using a 20x magnification objective, emphasizing the flow cell boundaries. Red (Rho-PE), green (CF) and merge channels are depicted for both A and B. (C) Z-stack image of the L1 sensor chip covered with POPC SUV labelled with Rho-PE and CF. YZ orthogonal projections were produced in order to evaluate the relative Z-position of the lipid membrane and CF probe. A vertical yellow line is located in the middle of the Z projection (40 μm). Red (Rho-PE), green (CF) and merge channels are depicted. A normalized Z plot profile is also shown locating the region of the lipid deposition. (D–H) FRAP analysis of the circular ROI according to the settings explained in the Supplementary Fig. S3. Rho-PE and CF fluorescent signals were collected from the ROI and normalized to the signal on the non-bleached area. The relative fluorescence recovery (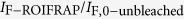 ) is thus shown to evaluate the lateral diffusion of the Rho-PE fluorescent lipids of the bilayer. Briefly, a time series of 4x Zoom 512 × 512 pixels images using a 20x magnification objective were taken: 10 frames were collected before bleaching of the 20 μm radius circular ROI (yellow) with 100 iterations (approx. 40 sec.) of 100% of 488, 514 and 561 laser intensity. After bleaching, 90–150 images were acquired according to the lipid mixture studied (D – POPC, E - POPC:Chol (2:1), F - POPC:Chol:SM (1:1:1), G - POPC:DPPC (1:1), H - POPC (control)) due to fluorescence recovery/sample burning percentage. Representative confocal images are presented in order of their acquisition time. Error bars correspond to the standard deviation of the mean. Experiments were performed in triplicate.
) is thus shown to evaluate the lateral diffusion of the Rho-PE fluorescent lipids of the bilayer. Briefly, a time series of 4x Zoom 512 × 512 pixels images using a 20x magnification objective were taken: 10 frames were collected before bleaching of the 20 μm radius circular ROI (yellow) with 100 iterations (approx. 40 sec.) of 100% of 488, 514 and 561 laser intensity. After bleaching, 90–150 images were acquired according to the lipid mixture studied (D – POPC, E - POPC:Chol (2:1), F - POPC:Chol:SM (1:1:1), G - POPC:DPPC (1:1), H - POPC (control)) due to fluorescence recovery/sample burning percentage. Representative confocal images are presented in order of their acquisition time. Error bars correspond to the standard deviation of the mean. Experiments were performed in triplicate.