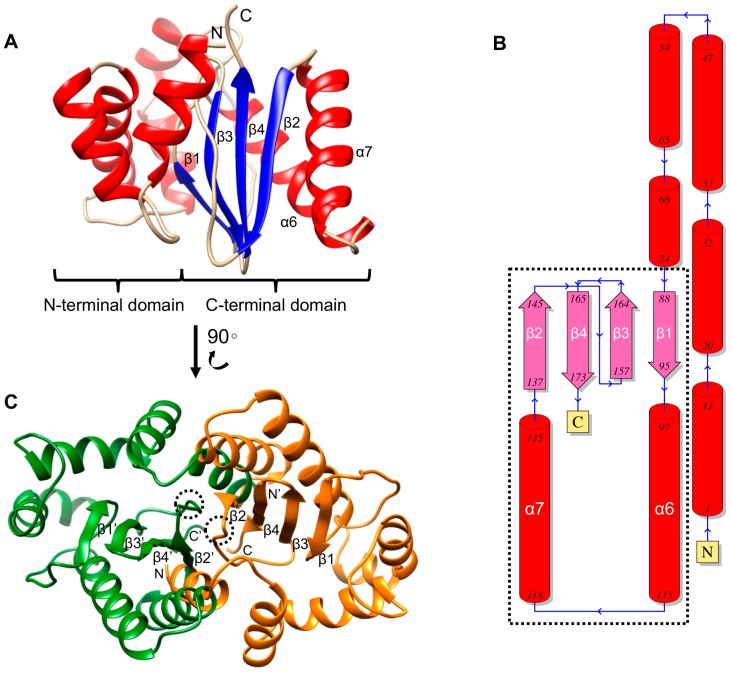
Figure 5.
Structural characterization and homodimer. (A) The α-helices, β-strands and coils of the CsTegu20.6 are colored in red, blue and gold, respectively. N- and C-terminal domains are connected by a flexible linker, which is an intrinsically disordered region. The C-terminal domain constitutes a sandwich of a four-stranded antiparallel β-sheet, β1-β3-β4-β2, and two α-helices, α6 and α7; (B) Secondary structure topology diagram showing domain composition and connectivity. The ribbon diagram indicates α-helices and β-strands in red and magenta color, respectively. The diagrams associated with the ferredoxin fold are indicated with a dotted rectangle; (C) The homodimer with symmetry-related CsTegu20.6 monomers (green and orange) was constructed by GalaxyGemini [44]. The missed fifth β-strand is indicated with dotted circles [45] (see Section 2.6).