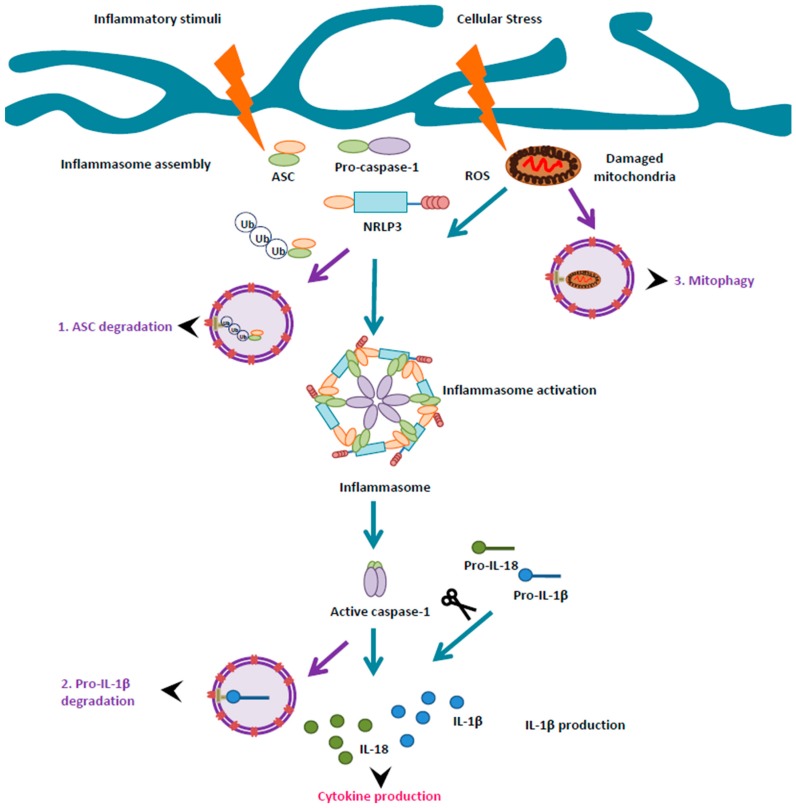
Figure 2.
Autophagy may negatively regulate microglial inflammation: potential mechanisms of inflammasome regulation. Inflammasomes are cytosolic macromolecular sensors that assemble after activation by infectious or damaging stimuli (illustrated by blue arrows). They consist of a ligand sensor (i.e., NLRP3), an adaptor protein (ASC), and the immature form of the inflammatory caspase, pro-caspase-1. Inflammasome assembly induces the proteolytic processing of pro-caspase-1 to its active form caspase-1. Subsequently, activated caspase-1 proteolytically processes the immature forms of inflammatory cytokines pro-IL-1β and pro-IL-18 to active inflammatory mediators IL-1β and IL-18. In peripheral macrophages, three types of modulatory interactions have been described to explain the suppresive effect of autophagy over the inflammasome (illustrated by purple arrows). Thus, autophagy may target (1) the inflammasome adaptor protein ASC and/or (2) the inflammasome substrate pro-IL-1β for digestion to the lysosome. On the other hand, autophagy may (3) selectively digest damaged, ROS-generating mitochondria by mitophagy. Note that none of these mechanisms have yet been described in microglia (see main text for details).