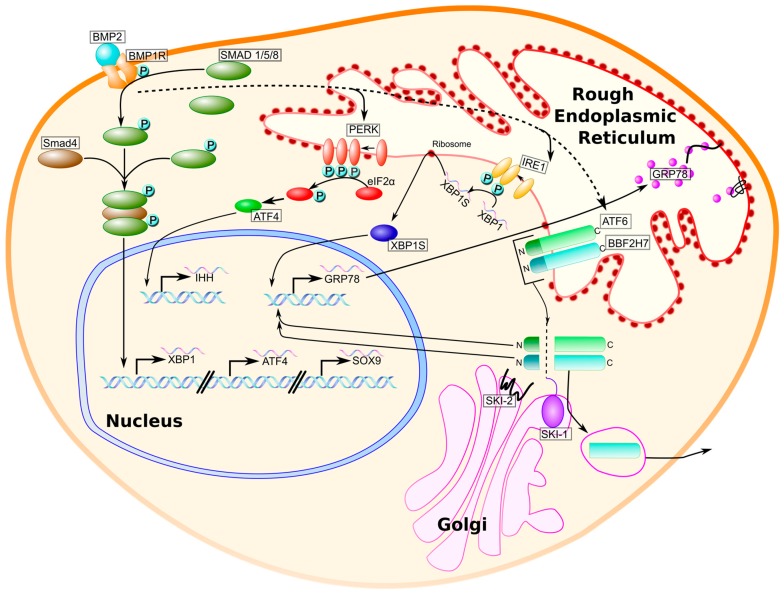
Figure 1.
BMP-mediated induction of physiological unfolded protein response (UPR) signaling in chondrocytes. Bone morphogenetic protein 2 (BMP2) is an extracellular growth factor that enacts UPR activity in chondrocytes in two ways: (1) the binding of BMP2 to its receptor, BMP1R, results in Smad 1/5/8 phosphorylation and the association with a co-Smad, Smad4, to form a trimeric activator of UPR genes XBP1, ATF4, and the chondrogenesis regulator SOX9 (solid line); and (2) BMP2 increases the activity of the UPR transducers PKR-like endoplasmic reticulum kinase (PERK), inositol-requiring endonuclease 1 (IRE1), activating transcription factor 6 (ATF6) (dotted line), and BBF2H7, an ATF6 homolog expressed by chondrocytes. PERK phosphorylates eukaryotic translation initiation factor 2a (eiF2a) to halt general protein translation while promoting the translation of activating transcription factor 4 (ATF4) (translation not shown). IRE1 splices XBP1 mRNA to generate XBP1S mRNA, which is translated to yield the transcription factor XBP1S. ATF6 and BBF2H7 translocate to the Golgi, where they are cleaved by site-1 and site-2 proteases (SKI-1 and SKI-2) to generate N-termini that serve as transcription factors for chaperone proteins, such as glucose-regulated protein 78 kDa (GRP78). The upregulation of chaperone proteins enhances the folding capacity of the ER during chondrogenesis.