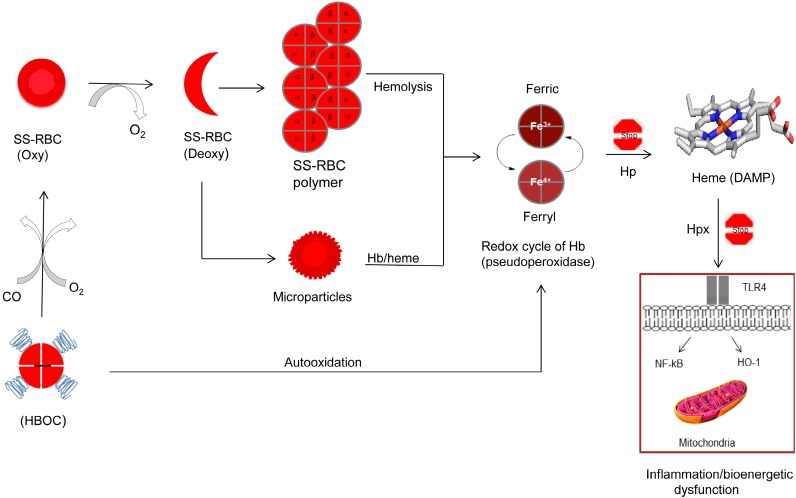
Figure 1.
Proposed antisickling functions of hemoglobin-based oxygen carriers (HBOCs) and possible complications of heme oxidative pathways. Red blood cell with predominately sickle cell hemoglobin (HbS) undergoes a vicious sickling and unsickling cycle in the microvasculature resulting in deoxyHbS fibers and free hemoglobin (Hb) spilling out of ruptured cells. Hb-laden microparticles release their contents (Hb and heme). Free Hb undergoes uncontrollable oxidation and oxidative changes through its classic pseudoperoxidase activity (redox cycling between ferric and ferryl heme) resulting ultimately in the loss of heme. Heme as damage associated molecular pattern (DAMP) molecule triggers Toll-like receptor 4 (TLR4), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and heme oxygenase-1 (HO-1) activation. This leads to inflammatory responses and altered cell metabolism, including mitochondrial dysfunction. Haptoglobin (Hp) (protein scavenger) and hemopexin (Hxp) (heme scavenger) can effectively inhibit Hb oxidative side reactions and heme toxicity. HBOCs due to their relatively smaller sizes can reach microvasculature and by delivering oxygen (O2) and/or carbon monoxide (CO) may reverse sickling. However, HBOCs may aggravate oxidative stress by initiating its own damaging oxidative pathways. SS RBCs: sickle cell red blood cells.