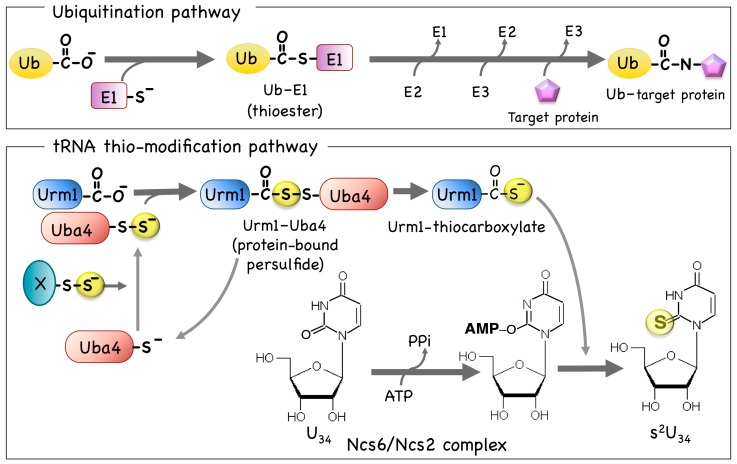
Figure 3.
The UBL—ubiquitin-activating enzyme (E1)-like protein system related to the thio-modification of cytosolic transfer RNA (tRNA). The protein modifier system of ubiquitination found in eukaryotes (upper column) is compared with a plausible model for the sulfur transfer between Uba4 and Urm1, which is for the s2U34 formation in yeast cytosolic tRNA (lower column). In the ubiquitination pathway, ubiquitin (Ub) is first bound to the UBA protein E1 (a sulfhydryl of an active cysteine residue is shown in a yellow circle) and then, after several reactions, is finally bound to form a thioester bond. In the thio-modification pathway, a UBL protein Urm1 is first bound to the partner UBA protein, Uba4. A complex of Ncs6 and Ncs2 (the Ncs6/Ncs2 complex) is thought to be involved in the last step of the tRNA recognition and thio-modification of cy-tRNAs. Sulfur atoms transferred in this system are shown with filled circles.