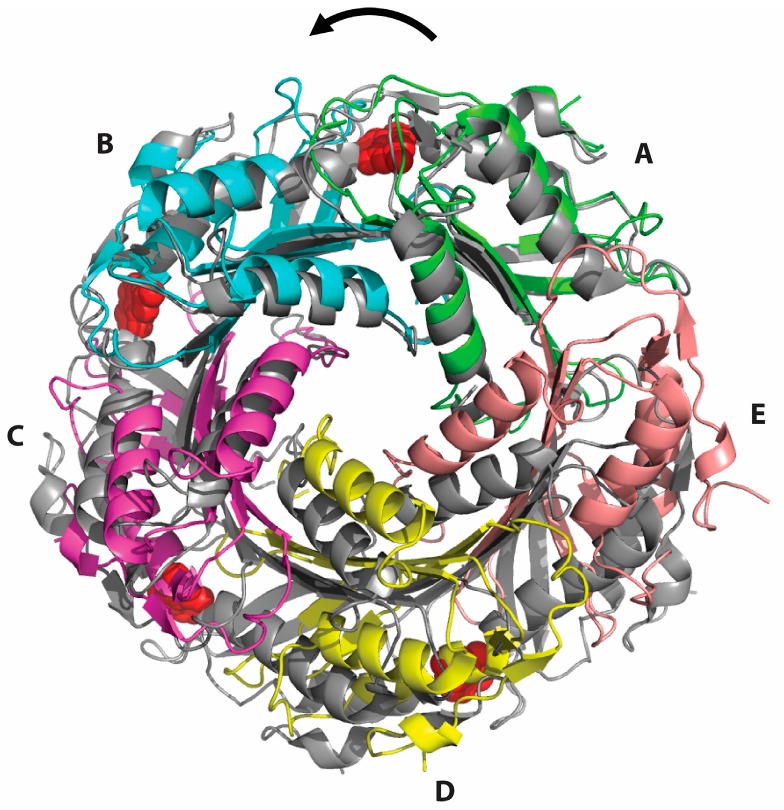
Figure 4.
Superposition of the crystal structures of the substrate-free Glu97Gln mutant of B. subtilis QueF (colors) with the substrate-bound wild-type enzyme as a thioimide intermediate (grey) generated by optimizing alignment of subunits A from both structures. Successive shifts in the positions of subunits B–E result in tightening of the decamer in a counterclockwise screw fashion indicated by the arrow. For clarity, only one pentamer is shown. Bound preQ0 molecules in the wild-type structure are shown in red.