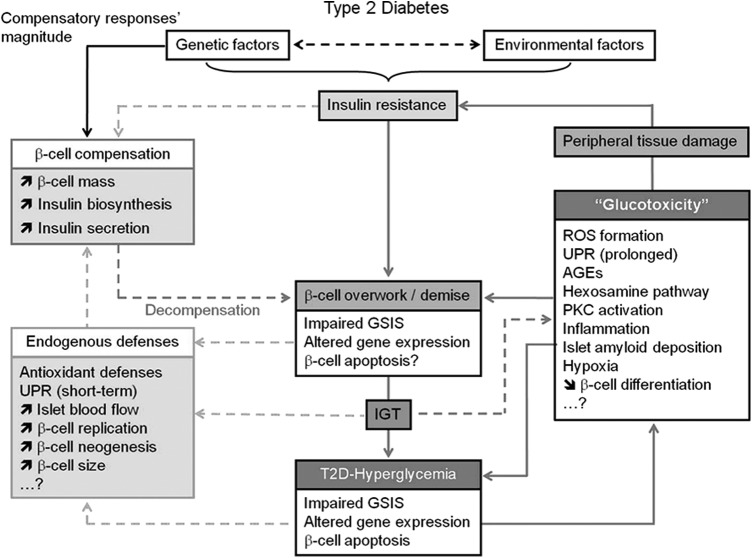
FIG. 4.
Schematic representation of the different mechanisms underlying beta-cell pathophysiology in T2D that precede and follow the establishment of hyperglycemia and the role of glucotoxicity in the aggravation of insulin resistance and beta-cell failure. In the absence of a defect in GSIS, beta cells maintain normoglycemia at the price of hyperinsulinemia. The extent of beta-cell compensation is thought to be predetermined genetically. This adaptation involves a coordinated increase in beta-cell mass, insulin biosynthesis, and insulin secretion. However, in genetically predisposed subjects, this phase is bypassed by a second phase of decompensation due to the inability of beta cells to sustain an adequate secretory response to match the organism demand (beta-cell overwork). This phase is characterized by the alteration of glucose-induced insulin secretion, gene expression and likely beta-cell apoptosis leading to the development of IGT, and finally the establishment of hyperglycemia with reduction of functional beta-cell mass. Chronic hyperglycemia leads to the exacerbation of beta-cell overwork and the alteration of beta-cell function and survival by several not fully understood mechanisms. In addition, hyperglycemia exerts toxic effects on peripheral tissues, which contribute to the aggravation of insulin resistance. Very importantly, beta-cell endogenous defenses are triggered in response to beta-cell failure and elevation of glycemia to restore the functional beta-cell mass. The imbalance between the protective effects of endogenous defenses and the deleterious effects of glucotoxicity is at the root of T2D pathology. [Reprinted from Bensellam et al. (12) with permission from Elsevier]. GSIS, glucose-induced insulin secretion; IGT, impaired glucose tolerance; T2D, type 2 diabetes mellitus.