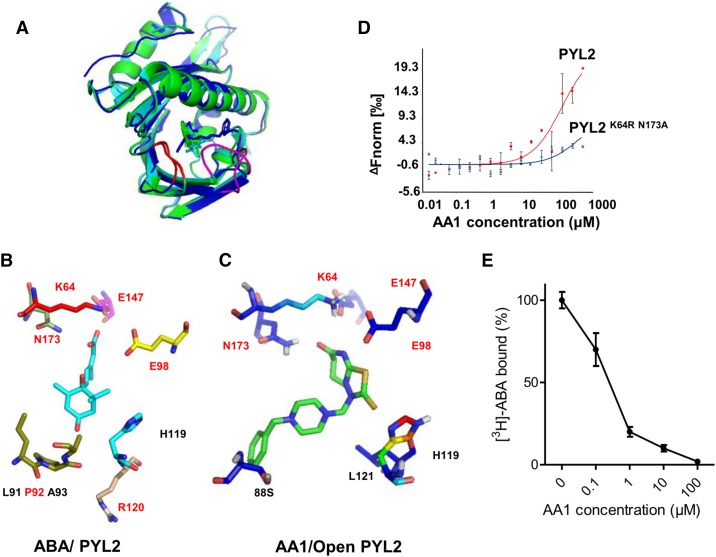
Figure 4.
Molecular docking of AA1 to PYL2. A, Overall structure of the AA1/PYL2 complex. The docking of AA1 to open PYL2 (PDB no. 3KDH) and closed PYL2 (PDB no. 3KDI) is shown. The AA1/closed PYL2 complex is in green; the AA1/open PYL2 complex is in blue; ABA is in cyan; the latch loop of PYL2 is in red; and the gate loop is in magenta. B, Network of ABA (cyan-stick model, with red oxygen atoms) inside the PYL2-binding pocket. The amino acids that form hydrogen bonds with ABA are in red. C, Network of AA1 (green-stick model, with red oxygen atoms, blue nitrogen atoms, and yellow sulfur atoms) inside the open PYL2-binding pocket. The amino acids that form hydrogen bonds with AA1 are in red. D, Mutations in key ligand-binding pocket residues of PYL2 compromise AA1 binding as determined by MST (n = 3; error bars = se). E, Competitive binding of [3H]ABA to PYL2 in the presence of AA1. The reactions were performed in the presence of 0.1 μm [3H]ABA and the indicated amounts of AA1. Each assay was replicated three times, and the results were normalized relative to no AA1 (n = 3; error bars = se).