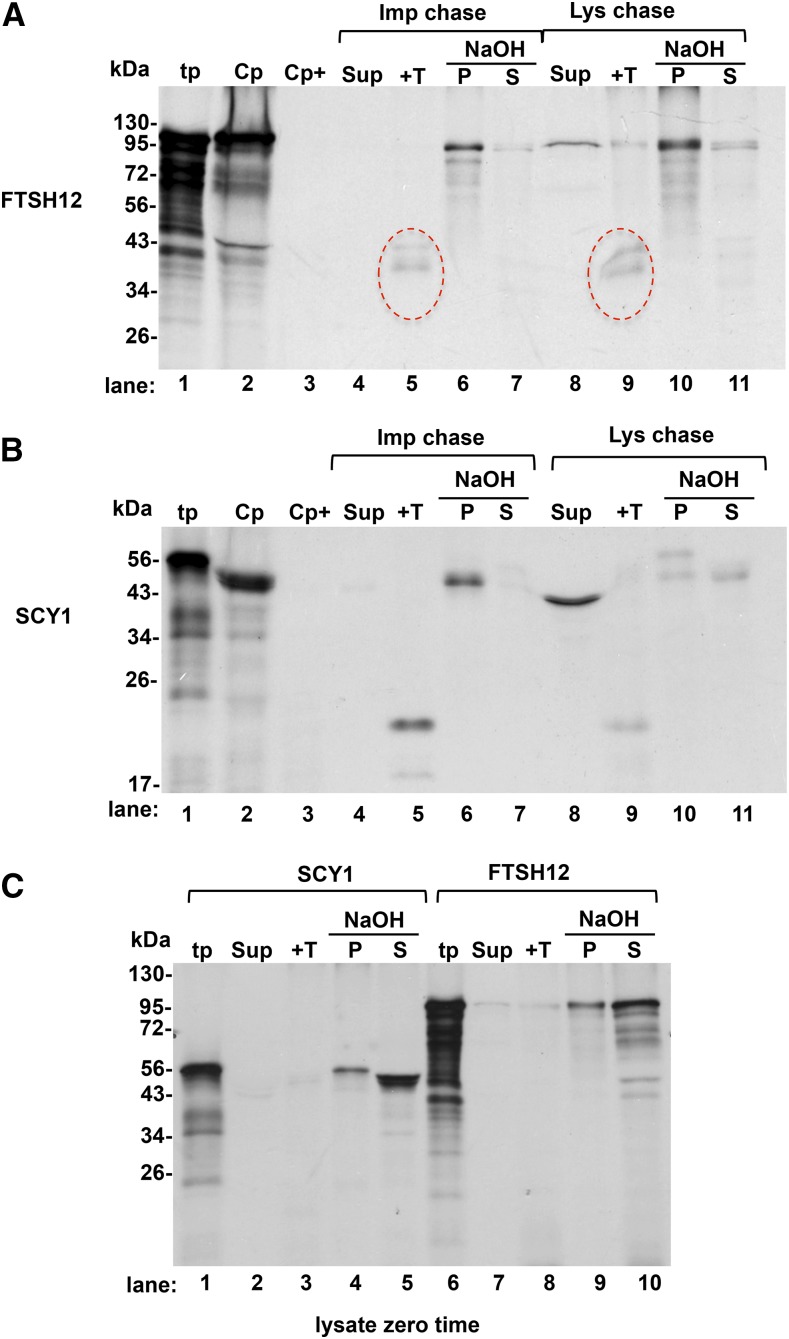
Figure 7.
FTSH12 is membrane integrated during a coupled import-integration bind-and-chase assay. The full precursors pre-FTSH12 and pre-SCY1 were translated in a homemade wheat germ system (see “Materials and Methods”). Translation products (tp) were diluted 15-fold in the final assay mixture to an estimated 0.1 mm ATP, which contained intact chloroplasts (Cp) at 0.33 mg mL−1 Chl. Precursor binding to chloroplasts (i.e. to the early intermediate stage; Schnell et al., 1994) was for 15 min at 25°C in darkness. Chloroplasts were recovered on Percoll cushions with (A and B, lane 3) or without (A and B, lane 2) thermolysin pretreatment. An aliquot of the untreated chloroplasts was subjected to a chase assay with 3 mm ATP (A and B, lanes 4–7). The remaining chloroplasts were lysed in 10 mm HEPES/KOH and adjusted to IB. A portion of the lysate was saved as a zero time control (C). The remainder was combined with ATP and 10× SE to 5 mm and 2.5× SE, respectively. Chase assays were for 30 min at 25°C in the light. Following the chase assays, the chloroplasts from the import chase were lysed, then this lysate as well as the lysate zero time and lysate chase were subfractionated and analyzed as follows: lysates were centrifuged to obtain a membrane fraction and a supernatant (Sup). One aliquot of membranes was treated with thermolysin (+T; lanes 5 and 9 in A and B and lanes 3 and 8 in C) and the other was extracted with 0.1 m NaOH as described (see “Materials and Methods”) into NaOH-resistant pellet (P) and NaOH-extracted supernatant (S). All gel-loaded samples were stoichiometrically equivalent. Red circle, degradation products from thermolysin digestion.