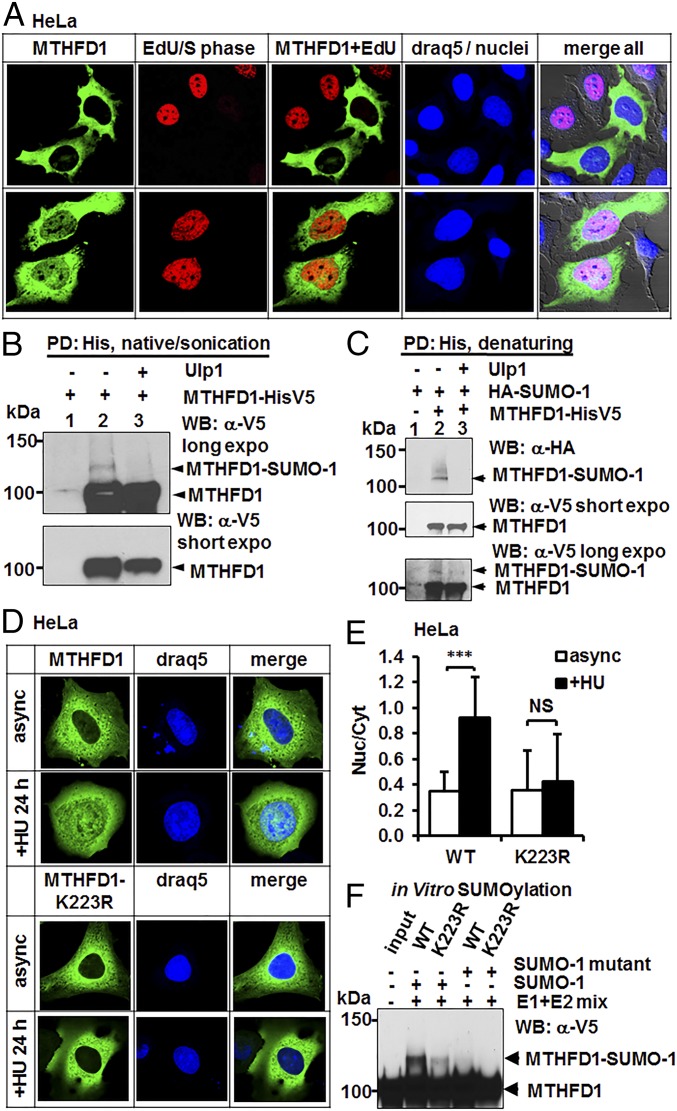
Fig. 2.
MTHFD1 translocates to the nucleus in S-phase and is a SUMOylated protein. (A) HeLa cells were transfected with MTHFD1-GFP and incubated with a nucleoside analog EdU for 4 h to label cells in S-phase. (Upper) MTHFD1-positive cells that did not incorporate the EdU label are also devoid of nuclear MTHFD1. (Bottom) MTHFD1-positive cells that incorporated the EdU label (red) simultaneously contain nuclear MTHFD1. (B) MTHFD1-HisV5 fusion protein was expressed in HeLa cells (lanes 2 and 3), and MTHFD1-HisV5 protein was Ni2+ affinity purified (pulldown, PD) from sonicated total cell lysate. Nontransfected HeLa cells were used as a negative control (lane 1). The MTHFD1 protein migrated at its predicted molecular mass (100 kDa), and an additional higher-molecular mass band was also observed (lane 2, MTHFD1-SUMO). After the pulldown, MTHFD1-HisV5 protein was treated with Ulp1 SUMO protease (lane 3), confirming the identity of the band as SUMOylated MTHFD1 protein. “Long” and “short” refer to relative film exposures. (C) HA-SUMO-1 construct, alone (lane 1) or together with MTHFD1-HisV5 construct (lanes 2 and 3), was transfected in HeLa cells, and MTHFD1-HisV5 protein was pulled down from denatured total cell lysate. After the pulldown and washes, isolated MTHFD1-HisV5 protein was treated with Ulp1 SUMO protease (lane 3), confirming the identity of the band as SUMOylated MTHFD1 protein. “Long” and “short” refer to relative film exposures. (D and E) MTHFD1 is SUMOylated on lysine residue 223. MTHFD1-GFP (WT) or MTHFD1-K223R-GFP fusion proteins were expressed in HeLa cells, and where indicated, cells were arrested in S-phase with 1 mM hydroxyurea for 24 h, fixed, and imaged. Representative images are shown. (E) Between 20 and 30 individual cells per condition were scored for nuclear to cytosolic ratio of MTHFD1 fluorescence intensity. Data are presented as mean ± SD, n > 20. Statistical significance was determined by Student t test. NS P ≥ 0.05, ***P < 0.001. (F) MTHFD1-HisV5 (WT) or MTHFD1-K223R-HisV5 proteins were expressed in HeLa cells and purified from HeLa lysate soluble fraction by Ni2+ affinity purification and used as substrates for in vitro SUMOylation reactions. MTHFD1-HisV5 (WT) (lane 2 and 3) or MTHFD1-K223R-HisV5 proteins were incubated with either SUMO-1 protein or a conjugation-incompetent SUMO-1 mutant protein (lane 4 and 5).