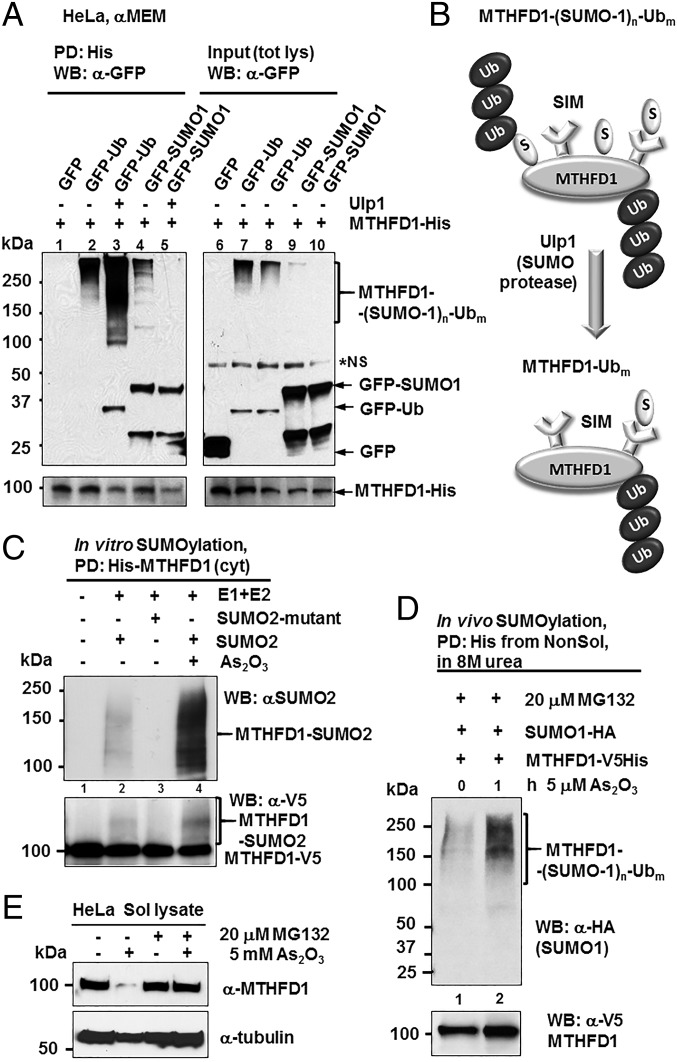
Fig. 3.
As2O3 targets MTHFD1 for increased SUMOylation and SUMO-dependent polyubiquitination and proteasomal degradation. (A) MTHFD1-HisV5 construct was cotransfected with vectors expressing GFP (lane 6), a GFP-Ub fusion protein (lanes 7 and 8), or a GFP-SUMO-1 fusion protein (lanes 9 and 10) in HeLa cells for 48 h. The expression of the transfected GFP fusion proteins and MTHFD1-HisV5 fusion protein was verified in total cellular lysates (tot lys) by α-GFP and α-V5 immunoblotting (input). MTHFD1-HisV5 fusion protein was purified by Ni2+ affinity pulldown (PD), followed by Ulp1 SUMO protease treatment (lanes 3 and 5). MTHFD1 SUMOylation and ubiquitination status was assessed by α-GFP immunoblot after MTHFD1 purification by Ni2+ pulldown. Nonspecific bands are labeled as NS. (B) The proposed model for MTHFD1 SUMOylation and ubiquitination. MTHFD1 ubiquitination is both SUMO-dependent and independent. SUMO interacts with MTHFD1 through both covalent and noncovalent interactions through the SUMO-interacting motif (SIM). (C) In vitro SUMOylation of purified MTHFD1 protein in the presence and absence of As2O3 (1 mM). (D) HeLa cells exposed to 5 μM As2O3 exhibited increased MTHFD1 SUMOylation in the presence of proteasomal inhibitor MG132 (E) MTHFD1 protein turnover in the presence or absence of MG132 and/or 5 mM As2O3 for 3 h.