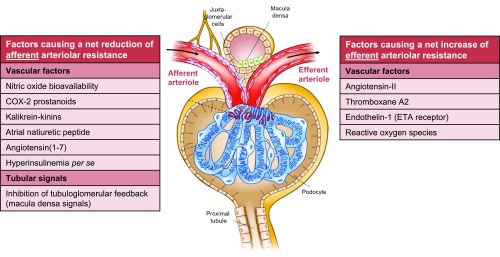
Figure 3.
Schematic (net) effect of factors implicated in the pathogenesis of glomerular hyperfiltration in diabetes. Several vascular and tubular factors32,48,123–126 are suggested to result in a net reduction in afferent arteriolar resistance, thereby increasing (single-nephron) GFR. Effects of insulin per se seem to depend on insulin sensitivity.96,97 A net increase in efferent arteriolar resistance—leading to increased GFR—is proposed for other vascular factors.32,42,71,124,127 Growth hormone128 and insulin-like growth factor-1129 likely increase filtration by augmenting total renal blood flow, without specific arteriolar preference. Glucagon and vasopressin seem to (principally) act through TGF.48 Intrinsic defects of electromechanical coupling or alterations in signal transduction in afferent arterioles may impair vasoactive responses to renal hemodynamic (auto)regulation.32 Augmented filtration by increases in the ultrafiltration coefficient, and net filtration pressure via reduction in intratubular volume and subsequent hydraulic pressure in Bowman’s space are not depicted. Several vascular factors may be released or activated after a (high-protein) meal (e.g., nitric oxide, cyclooxygenase-2 prostanoids, angiotensin II),48,50,130 whereas TGF becomes (further) inhibited, through increased amino acid- (and glucose) coupled sodium reabsorption in the proximal tubule49,50 and/or increased glucagon/vasopressin-dependent sodium reabsorption in the thick ascending limb.48 These changes may collectively play a part in postprandial hyperfiltration. COX-2, cyclooxygenase-2; ETA, endothelin A receptor.