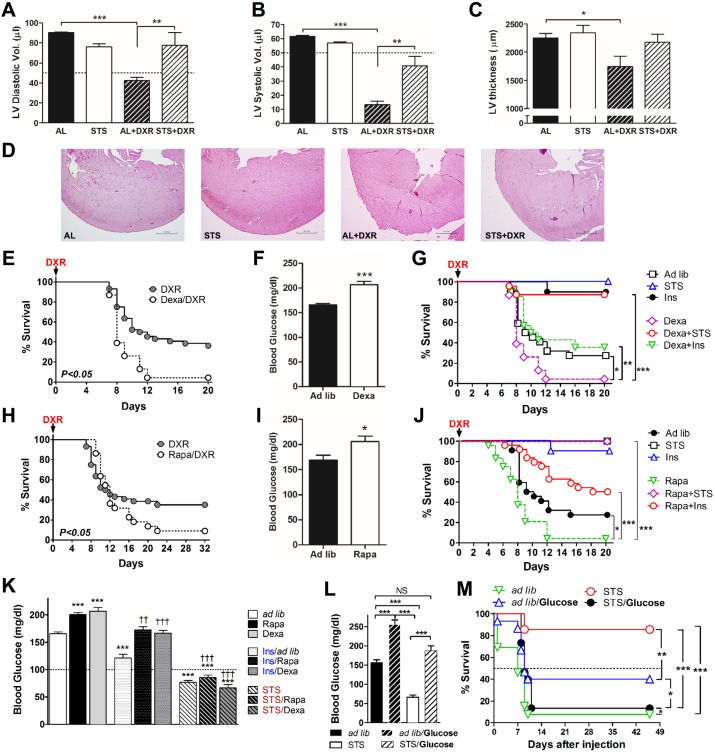
Fig 1. Short-term starvation (STS) and circulating-glucose reduction protect against doxorubicin (DXR)-induced cardiotoxicity.
(A, B) Echocardiographic and (C, D) histological analyses were performed following multiple DXR injections in order to assess the heart function. (A) Diastolic and (B) systolic left ventricle (LV) volume (n = 4 to 7) and LV wall thinning (C) (n = 6 to 9) of mice undergoing ad lib (AL), STS, DXR, and STS + DXR treatments are shown. Mice pretreated with dexamethasone (Dexa) (n = 24) or rapamycin (Rapa) (n = 22) were also injected with a high-dose DXR (24 mg/kg); survival and blood glucose levels (before DXR administration) are shown in E–F and H–I, respectively. (G, J) STS and insulin administration increased resistance against DXR toxicity and (K) reduced Dexa- and Rapa-induced hyperglycemia. (L) Mice were administrated glucose every 4 h for 24 h prior to DXR injection (24 mg/kg/mouse; intravenous [IV]) to directly increase blood glucose levels. Glucose levels were measured 1 h after the last glucose injection, at the same time of DXR administration. (M) Glucose-induced hyperglycemia prevented STS-induced protection (differential stress resistance [DSR]). Experiments were repeated twice. Data represent mean ± standard error of the mean (SEM). ANOVA (and Tukey post-analysis) was performed in A, B, C, K, and L; Student’s t test was performed in F and I; and log-rank test was performed on E, G, H, J, and M. p-values < 0.05 are considered significant (p-value < 0.05, 0.01, and 0.001 are indicated as *, *, and ***, respectively). Underlying data and method of statistical analysis are provided in S1 Data.