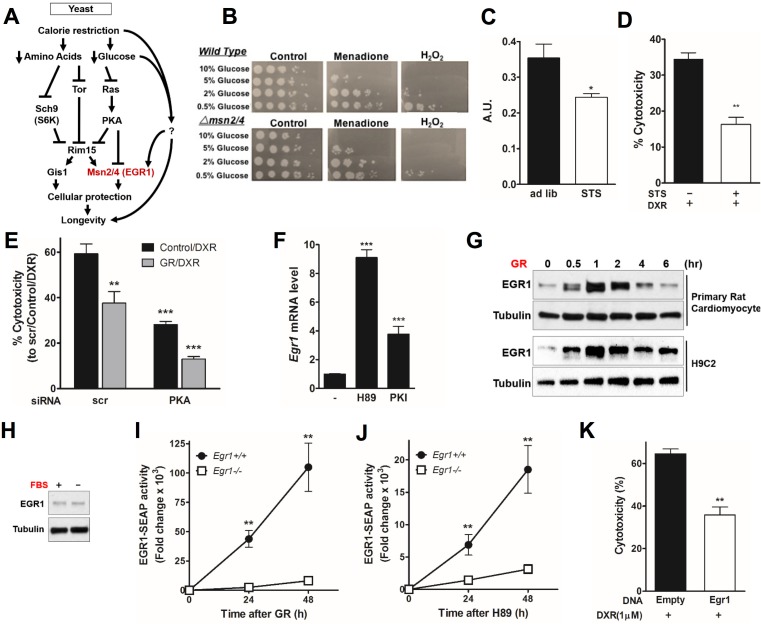
Fig 2. Glucose restriction (GR) induces early growth response protein 1 (Egr1) expression through protein kinase A (PKA)- and AMP-activated protein kinase (AMPK)-dependent regulation in cardiomyocytes.
(A) In yeast, nutrient-sensing pathways controlled by Sch9, Tor, and Ras/PKA converge on the downstream protein kinase Rim15. Transcription factors Msn2/4 and Gis1 enhance cellular protection through transactivation of stress response genes, leading to life span extension. (B) Four-day-old yeast cultured in different concentrations of glucose and treated with KPO4 buffer only, 200μM menadione, or 100mM H2O2 and incubated for 1 h before spotting in a 10-fold serial dilution on yeast extract peptone dextrose (YPD) plates. Spot test analysis reveals a heightened sensitivity to oxidative stress as glucose concentration increases. In addition, treatment with menadione and H2O2 resulted in a 5–10-fold increase in sensitivity in the Δmsn2/4 deletion background. (C) PKA kinase activity in cardiomyocytes was reduced in vitro by short-term starvation (STS) medium conditions for 48 h (n = 6) A.U.: arbitrary units. (D) Effect of STS on doxorubicin (DXR)-induced cardiotoxicity. Primary rat cardiomyocytes were incubated in STS or normal medium for 24 h and then treated with DXR (1 μM) for additional 24 h. Cytotoxicity was assessed by lactate dehydrogenase (LDH) release. (E) Cardiomyocytes were transfected with short interfering RNA (siRNA) against PKA (or a nonspecific control) at day 1, treated with GR or normal medium at day 2, and treated with DXR (1 μM) at day 3. Cytotoxicity is reported. (F) PKA inhibition triggers Egr1 expression. Primary rat cardiomyocytes were treated with PKA inhibitors, H89, or PKI for 1 h. mRNA expression level of Egr1 was measured. (G) Primary and H9c2 rat cardiomyocytes were incubated with GR medium for the indicated time and immunoblotted with an anti-EGR1 antibody. (H) Primary rat cardiomyocytes were incubated with serum-free medium for 1 h and immunoblotted with an anti-EGR1 antibody. (I, J) Egr1+/+ and Egr1-/- mouse embryonic fibroblasts (MEFs) transiently transfected with EGR1-secreted alkaline phosphatase (SEAP) reporter were incubated with (I) GR or (J) H89. SEAP activity was measured and quantified by using a chemiluminescent assay. (K) H9c2 cardiomyocytes transfected with a Flag-Egr1 expression vector were treated with DXR (1 μM) for 24 h. Cytotoxicity was measured by LDH release. Experiments were repeated three times, and the average for each technical repeat is reported in each graph (n = 3). The significance of the differences between experimental groups was determined by using one-way ANOVA (Tukey post-analysis test). Comparisons between groups were performed with Student’s t test. Data represent the mean ± SEM. p-values < 0.05 were considered significant (p-value < 0.05, 0.01, and 0.001 are indicated as *, *, and ***, respectively). Underlying data and method of statistical analysis are provided in S1 Data.