Abstract
Brain regions important for controlling movement are also responsible for rhythmic processing. In Parkinson disease (PD), defective internal timing within the brain has been linked to impaired beat discrimination, and may contribute to a loss of ability to maintain a steady gait rhythm. Less rhythmic gait is inherently less efficient, and this may lead to gait impairment including reduced speed, cadence, and stride length, as well as increased variability. While external rhythmic auditory stimulation (e.g. a metronome beat) is well-established as an effective tool to stabilize gait in PD, little is known about whether self-generated cues such as singing have the same beneficial effect on gait in PD. Thus, we compared gait patterns of 23 people with mild to moderate PD under five cued conditions: uncued, music only, singing only, singing with music, and a verbal dual-task condition. In our single session study, singing while walking did not significantly alter velocity, cadence, or stride length, indicating that it was not excessively demanding for people with PD. In addition, walking was less variable when singing than during other cued conditions. This was further supported by the comparison between singing trials and a verbal dual-task condition. In contrast to singing, the verbal dual-task negatively affected gait performance. These findings suggest that singing holds promise as an effective cueing technique that may be as good as or better than traditional cueing techniques for improving gait among people with PD.
Keywords: Gait, Cueing, Music, Singing, Parkinson disease
1. Background and Purpose
In Parkinson disease (PD), basal ganglia degeneration has been linked to impaired beat processing, as people with PD have difficulty discriminating beat-based rhythms [1–3]. This beat impairment may impact movement since brain regions involved in rhythm processing, such as the basal ganglia, cerebellum, premotor cortex, and supplementary motor area, are also responsible for motor function [4]. Neurodegeneration in these motor regions may disrupt the internal regulation of movement amplitude and timing in PD and lead to a loss of gait rhythmicity (i.e., ability to maintain a steady gait rhythm). While maintaining gait rhythmicity is an automatic and effortless process in healthy individuals, for people with PD, this becomes attention-demanding and is particularly impaired during performance of secondary tasks [5]. Less rhythmic gait is naturally more variable and less efficient, and may contribute to freezing of gait or falls [6, 7].
Music is well-established as an effective cueing technique to improve gait and restore gait rhythmicity [4, 8, 9]. Traditional auditory cueing, in which participants walk to a metronome beat or to the beat of a song, typically increases gait speed and elicits larger, more uniform steps [6, 9–11]. This technique, however, is challenging to implement consistently outside of the clinic because it requires use of an external device and headphones. The burden of wearing this device may prevent patients from using it regularly, particularly during short walking bouts in the home where falls commonly occur. Singing, on the other hand, requires nothing but one’s own voice. Additionally, most external cueing devices are set at a fixed tempo and incapable of adapting to a person’s varying cadence, thereby reducing effectiveness in the real world. One’s voice, in contrast, may be easily adapted to any circumstance, and may even help cue challenging gait situations such as step initiation, turning, or freezing. External cueing techniques have inconsistent carry-over effects, as the benefits of cueing are not always retained once the device is removed. Singing, however, is an active process that may cause melodies to get stuck in people’s heads and therefore may have longer lasting effects. Although external cueing devices may be effective at improving gait, they are not a perfect tool, and therefore, there is a need to find accessible and adaptive alternatives to traditional cueing techniques [12].
The purpose of our study was to determine if people with PD could generate their own cues through singing and if this novel cueing technique could improve gait in the same way that traditional cueing techniques do. Among the potential benefits of this technique are that it could be used at any time and in any place, without the need for a device to play music, and that it can be customized to match one’s cadence. Past research on imagined singing suggests the potential of singing to improve gait in PD and confirms that internal generation of musical cues is possible in PD and other neurological disorders [13–15]. However, no studies to date directly measure the effects of singing on gait parameters that have typically shown improvement with external cueing. Therefore, we developed a single-session protocol to test feasibility of singing as a tool to improve gait. We hypothesized that singing would stabilize gait in the same way that music does. We expected that singing would be as effective as traditional cueing at improving velocity, cadence, and stride length in PD, and that it would decrease gait variability as traditional cueing does. To assess the attentional demands of singing while walking, we also included a dual-task condition known to divide resources and cause gait decrement. We predicted that this verbal dual-task would be detrimental to gait, whereas singing would not.
2. Methods
2.1 Participants
Twenty-three individuals with PD were recruited from a convenience sample of people who were participating in a separate study [16] at Washington University School of Medicine (Table 1). Inclusion criteria were (1) a diagnosis of idiopathic PD, as determined by a board certified neurologist using diagnostic criteria for ‘definite PD’ [17], (2) ability to ambulate independently indoors for short distances without an assistive device, (3) absence of other neurologic disorder or dementia as measured by a minimum MMSE score of 24 [18], (4) absence of orthopedic injury or other comorbidity affecting gait, and (5) adequate vision and hearing (with or without a hearing aid). All participants gave informed consent to perform experimental procedures approved by the Human Research Protection Office at Washington University School of Medicine.
Table 1.
Participant Demographics.
| N | 23 (13 male) |
| Age, yrs (mean ± SD) | 69.5 (7.6) |
| MDS-UPDRS-III (mean ± SD) | 30.5 (11.8) |
| Hoehn & Yahr stage | II(10) |
| II.5(10) | |
| III(3) | |
| Years since Diagnosis (mean ± SD) | 3.8 (4.2) |
| MMSE (median, range) | 29 (24,30) |
2.2 Experimental Protocol
Participants were tested in the ‘on’ state (i.e., they had taken anti-Parkinson medication within the previous 2.5 hours) to maximize relevance to everyday walking conditions. Participants performed all walking trials on a 5m instrumented, computerized GAITRite Walkway (CIR Systems, Inc., Franklin, NJ). For all trials, participants were instructed to begin walking prior to reaching the GAITRite and to continue walking once off the mat to minimize acceleration and deceleration effects. An initial trial where participants were instructed to walk at their comfortable speed was used to determine each participant’s preferred cadence. This cadence was used to adjust song tempo to match each individual’s comfortable pace. Although cueing is often assessed using cues set to 110% of preferred cadence, we chose to use preferred cadence for this feasibility study to simplify task demands. For these musically-cued conditions, the cue was administered in the form of an instrumental version of “Row, Row, Row Your Boat” via a laptop no further than 10 m from the participant at any time during walking. Song tempo was adjusted for each individual using Audacity (The Audacity Team, audacity.sourceforge.net/download/) open source audio editing software. The song was chosen for its familiarity, as singing a life-long familiar melody results in better consolidation and higher retention [19] and because improvements in velocity and stride length have been seen in people with PD when synchronizing to a highly familiar song [20]. The particular instrumental version was selected for its high beat saliency, which enabled participants to more easily find the beat and sing along [21]. Follow-up interviews confirmed that all participants were able to hear the music and knew the melody and lyrics.
Participants completed three walking trials in each of five conditions as described below and were instructed to begin each trial when ready. Dual-task data were collected first as this was required as part of the study protocol for the larger trial. All other conditions were randomized to eliminate any training effects.
1. Uncued
This condition was used to represent ‘normal’ walking and provided a point of comparison for the other conditions. Participants were asked to walk at their preferred walking speed when given the signal to go. This occurred in silence as no cueing was present. In instances where the UNCUED condition came after a condition in which music played, participants were instructed not to think of the previously heard song as they walked.
2. Music only (MUS)
Our music-only condition represents traditional cueing techniques in which music was playing and participants were asked to walk to the beat. Once the song was turned on for each trial, participants were told to take as long as needed to listen to the song, pick out the beat and begin walking.
3. Singing only (SING)
Participants were asked to sing aloud while walking without music playing. In the absence of an external cue, participants were required to internally generate and produce the music to cue their walking. Therefore, this condition represented the novel cueing technique in which we were most interested.
4. Singing along with music (MUS+SING)
Participants were asked to walk to the beat of the music while singing along. Instructions for this condition were the same as for the MUS condition except that participants were now asked to sing aloud to the music. This condition was included to capture the potentially additive effect of listening to music while also singing.
5. Verbal dual-task condition (DT)
This is a commonly used dual task in which participants were asked to walk at preferred speed while generating as many words as possible that began with different letters of the alphabet (H, L, T). Participants were given instructions on this task and a letter was given just before they began walking so they did not have time to think of words in advance. At the end of the walkway, they turned around and repeated the protocol with the next letter.
Additional Measures
Disease severity was assessed by a trained physical therapist using the Movement Disorders Society Unified Parkinson’s Disease Rating Scale Motor Subscale 3 (MDS-UPDRS III) and Hoehn and Yahr staging (H&Y), the New Freezing of Gait Questionnaire (nFOGq) was used to assess freezing, and the Mini-mental Status Exam (MMSE) was used to assess cognition. Beat processing impairment was assessed by the Beat Alignment Test (BAT).
2.3 Data Analysis
IBM SPSS Statistics 22 was used for all statistical analyses. For each participant, data were averaged across the three trials of each condition. Normalized velocity, cadence, stride length, and variabilities of step time, single support time, and stride length were compared across conditions using one-way repeated measures ANOVAs. Variabilities were calculated as the standard deviation of each trial and then averaged across trials. Comparisons between the single initial trial used to determine preferred cadence and the three uncued trials were not statistically significant, and therefore we used the average of uncued trials to represent baseline. Post-hoc pairwise comparisons were used as appropriate, and Bonferroni corrections were used to correct for multiple comparisons. Statistical significance was set at p<0.05.
3. Results
3.1 Normalized velocity, cadence, and stride length
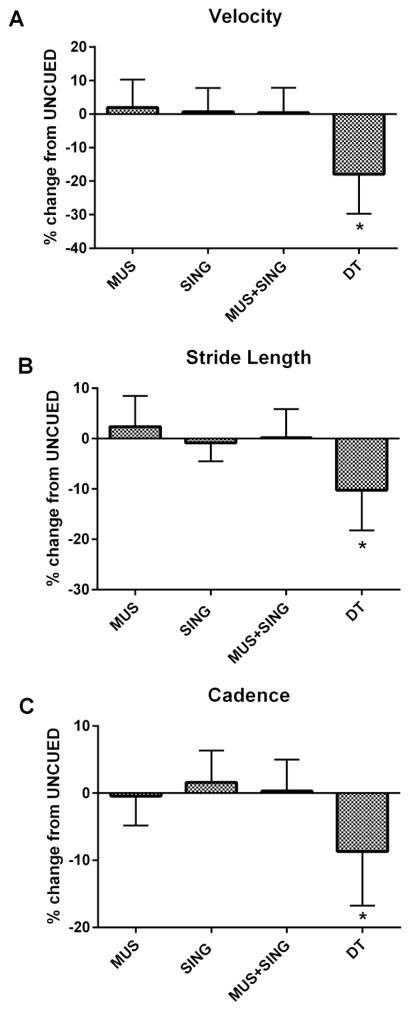
Cueing in the form of MUS, SING, or MUS+SING did not alter velocity, cadence, or stride length relative to UNCUED (Figure 1). DT, however, elicited significant decreases in normalized velocity (F(4,19)=16.418, p<.001), cadence (F(4,19)=7.04, p=.001), and stride length (F(4,19)=10.115, p<.001) compared to all other conditions (Table 2).
Figure 1.
Gait characteristics across 4 conditions as percent change from UNCUED walking. Error bars represent ± SEM, * denotes p<.001 where DT was worse than all other conditions.
Table 2.
Measures of gait velocity, stride length, and cadence across all 5 conditions.
| UNCUED | MUS | SING | MUS+SING | DT | |
|---|---|---|---|---|---|
|
|
|||||
| Velocity (cm/sec) | 123.97 (18.99) | 126.65 (23.74) | 124.87 (29.92) | 124.92 (24.29) | 101.75 (20.77)* |
| Stride Length (cm) | 131.4 (16.9) | 134.7 (20.2) | 130.4 (18.4) | 131.9 (20.7) | 118.1 (18.8)* |
| Cadence (steps/min) | 113.6 (9.7) | 113.1 (10.3) | 115.3 (9.6) | 113.9 (10.6) | 104.0 (14.3)* |
Values are means +/− SD.
denotes p<.001 where DT was worse than all other conditions.
3.2 Variability of step time, single support time, and step length
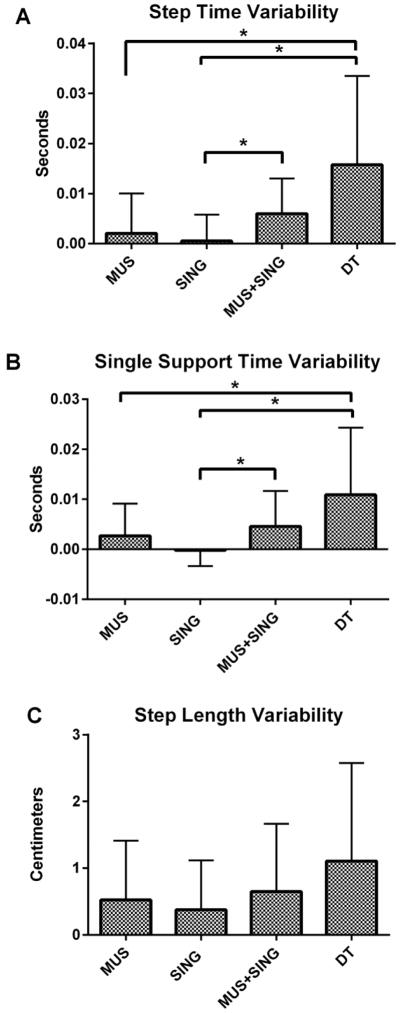
Variability measures revealed greater differences between cueing techniques. SING closely resembled UNCUED in that it showed minimal variability across all measures. Variability was significantly lower for SING compared to MUS+SING and DT for step time (F(4,19)=7.172, p=.008, F(4,19)=7.172, p=.003, respectively) and single support time (F(4,19)=6.806, p=.031, F(4,19)=6.806, p=.004, respectively). Step length revealed a similar but non-significant trend in which SING was less variable than other cued conditions. MUS+SING was associated with higher gait variability than all other cued conditions and this was significant for step time when compared to UNCUED (F(4,19)=6.806, p=.045) (Figure 2). The DT condition was the most variable of all five conditions. For step time, DT was more variable than UNCUED (F(4,19)=7.172, p=.003), MUS (F(4,19)=7.172, p=.021), and SING (F(4,19)=7.172, p=.003). For single support time, DT was more variable than UNCUED (F(4,19)=6.806, p=.003) and SING (F(4,19)=6.806, p=.004) (Table 3).
Figure 2.
Gait variability across 4 conditions as compared to UNCUED walking. Data represent standard deviations ± SEM. * denotes significance of p<.05.
Table 3.
Measures of gait variability across 5 conditions.
| UNCUED | MUS | SING | MUS+SING | DT | |
|---|---|---|---|---|---|
|
|
|||||
| Step time SD | 0.018 (.005)* | 0.020 (.008)* | 0.018 (.005)*# | 0.024 (.007) | .034 (.018) |
| Single support time SD | 0.015 (.005)* | 0.018 (.006) | 0.015 (.003)*# | 0.020 (.007) | 0.026 (.013) |
| Step length SD | 2.517 (0.548) | 3.041 (0.750) | 2.894 (0.953) | 3.167 (1.271) | 3.624 (1.130) |
Values are standard deviations (SD) ± SEM. Significance is set at p<0.05.
denotes significantly better than DT.
denotes significantly better than MUS+SING.
4. Discussion
In this study, we explored a novel cueing technique to improve gait in PD by singing a song oneself rather than listening to a song, as in traditional cueing techniques. Our primary finding is that singing at a tempo matching comfortable gait pace may improve gait variability while not causing other gait decrements. The absence of gait decrements during singing trials indicates that singing while walking was not excessively demanding for people with PD. This was further supported by the comparison to a verbal dual-task condition which negatively affected gait performance, whereas singing did not. In addition, singing while walking produced less variability than other cueing techniques. Variability is a valuable marker of overall gait performance that reflects gait unsteadiness and dyscontrol. People with PD have increased gait variability which reflects reduced automaticity of walking [22]. Stride-to-stride fluctuations related to both stride time and stride width are sensitive measures that correlate more closely to fall risk than other elements of gait [23]. Therefore, decreasing gait variability may be even more important than increasing gait speed or distance. Our results suggest that singing holds promise as a cueing technique that may be as beneficial as traditional cueing techniques for improving gait in PD.
Singing is already widely used as a therapeutic technique for voice rehabilitation in PD because it targets hypophonia, a common PD symptom, and elicits improvements in speech intelligibility, vocal intensity, and respiratory function [24, 25]. However, it is not known if the benefits of singing may extend beyond speech to improvements in motor control. External auditory cueing through music is widely established as an effective tool to stabilize gait in PD [4, 9]. The musical cue may work by replacing the defective internal timing mechanism within the basal ganglia with an external template to which people can match their movement [9, 10]. By contrast, little is known about whether singing can serve the same purpose or if impaired beat processing would preclude people with PD from either creating an internal template through song or synchronizing movement to it.
We expected some of our participants would be unwilling or unable to sing aloud; however, all participants sang aloud with apparent ease. Ability to do the task was likely not attributable to musical experience, as only nine participants reported having any musical training. In addition, our participants were a subset of a larger sample that showed impaired beat processing as compared to controls [26], confirming past reports among people with PD [27]. Our results support the idea that, in spite of this deficiency, people with PD can internally generate music and use it as a cue to guide movement, as was shown previously in a study in which imagined singing was used to improve motor timing in people with PD [13].
When comparing singing trials to the verbal dual-task condition, we noted significant differences in all gait measures. Word generation created a dual-task effect that slowed and destabilized gait. This corroborated previous studies where gait impairment was exacerbated during a concurrent speaking task in people with PD [28, 29]. Dividing limited cognitive and motor resources between complex activities is known to disrupt gait automaticity and increase stride-to-stride variability [5]. Our finding that singing did not negatively affect gait suggests that singing a rhythmic and familiar song may not divide resources in the same way as speaking.
When comparing cueing techniques, we noted that walking to music, either while listening, as in traditional cueing, or while singing along, increased variability of temporal and spatial gait parameters. These increases were not byproducts of changes in speed, cadence, or stride length, as these measures were unchanged. In the singing only condition, by contrast, no music was present so participants did not have to match their singing or footsteps to an external source. Higher variability in the musically-cued conditions may reflect the extra attentional resources required to synchronize even simple, automatic movements to sound [14]. Thus, participants may have had an easier time walking to the beat when they were able to generate the song themselves than when they had to synchronize to music. Another possibility is that active music-making (such as singing) may confer greater motor benefits than passive music listening [30] by affecting movement “vigor” or eagerness to move. While synchronizing movement to music induces an arousal effect that makes movement faster, larger, and more vigorous [31] and can lead to greater motor network activation [14], synchronizing movement to one’s own voice may elicit an even stronger motor response, or at least a more precisely timed one.
Singing may hold other benefits over external auditory cueing. Studies suggest that adaptive cues that synchronize to an individual’s walking speed are more effective than set-tempo cues, and singing, similarly, can be altered to fit any situation [12]. Singing also creates a longer-lasting memory trace over spoken words, resulting in improved memory consolidation and retention [32]. Our participants reported that the song got “stuck in their heads”, possibly reflecting carry-over benefits and supporting the theory that singing mentally after singing aloud allows rhythm recall and facilitates movement [33]. Singing may also be useful in challenging gait situations that cause freezing, as one’s voice can easily be turned on and off as needed. Six participants in our sample were identified as freezers, and some of them suggested singing might be helpful during freezing episodes. This is promising as auditory cueing has been shown to benefit freezers and non-freezers alike [34, 35]. Singing, therefore, may be feasible for a wide variety of patients in a variety of situations.
Several limitations of our study are noted. One is that our singing and dual-task paradigms were not equally demanding, as participants sang a familiar song but spoke a word-generation task that likely required higher cognitive effort. Another is that we took no explicit measures of attention, so we cannot know how division of resources differs when synchronizing movement to endogenous cues versus heard cues. Also, since we tested only one version of one song, we cannot rule out the possibility that another song, or one without lyrics, may have elicited a different response. A potential criticism of this technique is that singing aloud may not be preferred to wearing an external cueing device for people who experience gait difficulty in public settings. Therefore, future work should examine the possibility that imagined singing, or a combined training program that included both audible and mental singing, could ameliorate gait in the same way as singing aloud.
In conclusion, singing positively affected gait variability while having no detrimental effect on velocity, cadence, or stride length. Whereas traditional cueing techniques require the use of external devices that typically do not adapt to one’s cadence and do not convey long-lasting benefits in their absence, singing can be easily implemented anytime, anywhere, without the need for significant training, and could therefore be translated into practice quite expeditiously. There is a strong need for inexpensive, non-invasive, and widely accessible interventions to address gait impairments in PD. Singing holds promise as a useful alternative to traditional cueing techniques to regulate gait in PD. Further study is warranted to determine the effect of singing tempo on gait, how long the effects of singing last, and who is most likely to benefit from this novel technique.
Highlights.
Singing had no detrimental effect on velocity, cadence, or stride length.
Singing positively affected gait variability.
A verbal dual-task condition slowed and destabilized gait, whereas singing did not.
Singing holds promise as an effective cueing technique for people with PD.
Acknowledgments
The authors thank Ryan Duncan, Martha Hessler, and Rich Nagel for assistance with data collection. This work was supported by NIH R01NS077959, the Program in Physical Therapy at Washington University, the Greater St. Louis Chapter of the American Parkinson Disease Association (APDA) and the APDA Center for Advanced Parkinson Disease Research at Washington University.
Footnotes
Conflict of interest statement:
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harrington DL, Haaland KY, Knight RT. Cortical networks underlying mechanisms of time perception. J Neurosci. 1998;18(3):1085–95. doi: 10.1523/JNEUROSCI.18-03-01085.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grahn JA. The role of the basal ganglia in beat perception: neuroimaging and neuropsychological investigations. Ann N Y Acad Sci. 2009;1169:35–45. doi: 10.1111/j.1749-6632.2009.04553.x. [DOI] [PubMed] [Google Scholar]
- 3.O’Boyle DJ, Freeman JS, Cody FW. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain. 1996;119(Pt 1):51–70. doi: 10.1093/brain/119.1.51. [DOI] [PubMed] [Google Scholar]
- 4.Nombela C, et al. Into the groove: can rhythm influence Parkinson’s disease? Neurosci Biobehav Rev. 2013;37(10 Pt 2):2564–70. doi: 10.1016/j.neubiorev.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Lohnes CA, Earhart GM. The impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson disease. Gait Posture. 2011;33(3):478–83. doi: 10.1016/j.gaitpost.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Rochester L, et al. The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson’s disease. Arch Phys Med Rehabil. 2005;86(5):999–1006. doi: 10.1016/j.apmr.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff JM, et al. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149(2):187–94. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- 8.Wittwer JE, Webster KE, Hill K. Music and metronome cues produce different effects on gait spatiotemporal measures but not gait variability in healthy older adults. Gait Posture. 2013;37(2):219–22. doi: 10.1016/j.gaitpost.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Thaut MH, et al. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov Disord. 1996;11(2):193–200. doi: 10.1002/mds.870110213. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff JM, et al. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur J Neurosci. 2007;26(8):2369–75. doi: 10.1111/j.1460-9568.2007.05810.x. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwboer A, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78(2):134–40. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hove MJ, Keller PE. Impaired movement timing in neurological disorders: rehabilitation and treatment strategies. Ann N Y Acad Sci. 2015;1337:111–7. doi: 10.1111/nyas.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoh M, Kuzuhara S. Training in mental singing while walking improves gait disturbance in Parkinson’s disease patients. Eur Neurol. 2008;60(5):237–43. doi: 10.1159/000151699. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer RS, et al. Moving to music: effects of heard and imagined musical cues on movement-related brain activity. Front Hum Neurosci. 2014;8:774. doi: 10.3389/fnhum.2014.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schauer M, Mauritz KH. Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clin Rehabil. 2003;17(7):713–22. doi: 10.1191/0269215503cr668oa. [DOI] [PubMed] [Google Scholar]
- 16.Earhart GM, et al. Comparing interventions and exploring neural mechanisms of exercise in Parkinson disease: a study protocol for a randomized controlled trial. BMC Neurol. 2015;15:9. doi: 10.1186/s12883-015-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Racette BA, et al. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999;88(5):539–43. [PubMed] [Google Scholar]
- 18.Dalrymple-Alford JC, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–25. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 19.Moussard A, et al. Learning sung lyrics aids retention in normal ageing and Alzheimer’s disease. Neuropsychol Rehabil. 2014;24(6):894–917. doi: 10.1080/09602011.2014.917982. [DOI] [PubMed] [Google Scholar]
- 20.de Bruin N, et al. Walking with music is a safe and viable tool for gait training in Parkinson’s disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinsons Dis. 2010:483530. doi: 10.4061/2010/483530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancela J, et al. Designing auditory cues for Parkinson’s disease gait rehabilitation. Conf Proc IEEE Eng Med Biol Soc; 2014; 2014. pp. 5852–5. [DOI] [PubMed] [Google Scholar]
- 22.Baker K, Rochester L, Nieuwboer A. The effect of cues on gait variability--reducing the attentional cost of walking in people with Parkinson’s disease. Parkinsonism Relat Disord. 2008;14(4):314–20. doi: 10.1016/j.parkreldis.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haneishi E. Effects of a music therapy voice protocol on speech intelligibility, vocal acoustic measures, and mood of individuals with Parkinson’s disease. J Music Ther. 2001;38(4):273–90. doi: 10.1093/jmt/38.4.273. [DOI] [PubMed] [Google Scholar]
- 25.Narayana S, et al. Neural correlates of efficacy of voice therapy in Parkinson’s disease identified by performance-correlation analysis. Hum Brain Mapp. 2010;31(2):222–36. doi: 10.1002/hbm.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron DJ, et al. The Effect of Dopaminergic Medication on Beat-Based Auditory Timing in Parkinson’s Disease. Front Neurol. 2016;7:19. doi: 10.3389/fneur.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grahn JA, Brett M. Impairment of beat-based rhythm discrimination in Parkinson’s disease. Cortex. 2009;45(1):54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Baker K, Rochester L, Nieuwboer A. The immediate effect of attentional, auditory, and a combined cue strategy on gait during single and dual tasks in Parkinson’s disease. Arch Phys Med Rehabil. 2007;88(12):1593–600. doi: 10.1016/j.apmr.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Yogev G, et al. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22(5):1248–56. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 30.Pacchetti C, et al. Active music therapy in Parkinson’s disease: an integrative method for motor and emotional rehabilitation. Psychosom Med. 2000;62(3):386–93. doi: 10.1097/00006842-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Leman M, et al. Activating and relaxing music entrains the speed of beat synchronized walking. PLoS One. 2013;8(7):e67932. doi: 10.1371/journal.pone.0067932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons-Stern NR, Budson AE, Ally BA. Music as a memory enhancer in patients with Alzheimer’s disease. Neuropsychologia. 2010;48(10):3164–7. doi: 10.1016/j.neuropsychologia.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buetow SA, et al. Conceptualizing how group singing may enhance quality of life with Parkinson’s disease. Disabil Rehabil. 2014;36(5):430–3. doi: 10.3109/09638288.2013.793749. [DOI] [PubMed] [Google Scholar]
- 34.Willems AM, et al. The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study. Disabil Rehabil. 2006;28(11):721–8. doi: 10.1080/09638280500386569. [DOI] [PubMed] [Google Scholar]
- 35.Chuma T, et al. Motor learning of hands with auditory cue in patients with Parkinson’s disease. J Neural Transm. 2006;113(2):175–85. doi: 10.1007/s00702-005-0314-4. [DOI] [PubMed] [Google Scholar]