INTRODUCTION
Primary Open Angle Glaucoma (POAG) is the most common form of glaucoma in the US. This complex inherited disorder with different prevalence rates in various racial groups accounts for almost 70% of all glaucoma cases globally [1]. Although POAG is a leading cause of vision loss, and thus a serious clinical and public health problem, information on established risk factors for the disease is limited. There is compelling evidence that genetic factors play a central role in the pathophysiology of POAG. The risk of POAG in first-degree relatives of affected individuals is 7–10 times greater than in the general population [2, 3], and a high concordance has been observed between monozygotic twins [4]. A number of different genes appear to be involved in the etiology of POAG. Studies linking POAG to at least 15 different genomic regions (see OMIM GLC1A-GLC1P) substantiate the polygenic nature of this disease. Additionally, specific genes (like myocilin and optineurin) have also been identified although they account for a small number of glaucoma cases. Furthermore, despite the number of studies investigating non-genetic risk factors, only race, older age, intraocular pressure (IOP) and corneal thickness have been consistently identified [5–9]. Of these only IOP is modifiable and is currently the only means we have to clinically treat the disease.
Even when causative mutations are present in specific individuals though, penetrance is variable [10]. In addition many of the risk factors that predispose to the development of glaucoma (like high IOP) seem to behave like quantitative locus traits [11, 12]. That suggests that environmental factors may influence glaucoma development and progression [13–15]. Despite that, prior work has failed to detect strong environmental risk factors. In large epidemiological studies common broad environmental factors (like smoking) or personal characteristics (like BMI) failed to show an association with disease risk [9]. However, analysis of subgroups within very large cohorts has detected associations with estrogen status in POAG [16, 17] and with sunlight exposure in pseudoexfoliation glaucoma although the risk imparted by these factors is at best modest [18, 19].
Based on a literature review to identify other possible factors (see for example [20]) that may potentially affect neurodegeneration combined with personal clinical observations (JD), we decided to investigate a potential association between dental health and POAG. In addition, because of laboratory data pointing towards a role of peripheral inflammation in glaucoma we further investigated whether specific commensal organisms in the oral cavity may be correlated with disease status or disease severity [21].
Our hypothesis was that chronic inflammation linked to the oral microbiome, and manifesting as worse dental health, is a potentially modifiable risk factor associated with chronic open angle glaucoma. To explore this novel hypothesis, we conducted a pilot case control study to compare dental health data and levels of different bacterial strains from the ocular microbiome in glaucoma cases and controls without glaucoma.
METHODS
This case-control study adhered to the tenets of Declaration of Helsinki and was conducted in compliance with HIPAA regulations. All protocols involving human subjects were approved by the Institutional Review Board of SUNY Downstate Medical Center prior to study initiation. Signed informed consent was obtained from all subjects.
Subject recruitment and sample collection
Subjects enrolled in this study were part of a larger cohort of subjects enrolled into a study of gene and environment interactions in glaucoma. Subjects with open angle glaucoma (n=119) as well as a control group without glaucoma (n=78) were recruited for the current study from the SUNY Downstate Eye clinics between 2010 and 2013. All potentially eligible individuals, 40 years and older seen at one of the SUNY Downstate Eye clinics on specific days were selected by the examining physician for possible participation in this study. Eligibility for glaucoma cases required meeting the following clinical and visual field criteria 1) having open angles (at least grade III Shaffer grade), 2) the presence of a characteristic glaucomatous visual field defect (e.g. arcuate defects or nasal steps respecting the horizontal midline or more advanced visual field loss (like central or temporal islands in the absence of other ocular pathology) on at least one Humphrey visual field and 3) typical optic nerve head (ONH) cupping (cup to disc ratio (CDR) >0.8) in at least one eye based on clinical examination. In addition, all cases were either using IOP lowering agents or had undergone glaucoma surgery. Control group eligibility required having no current or past IOP elevation (IOP<21 mmHg), no prior use of IOP lowering medications, no significant ONH asymmetry (CDR difference < 0.2) and had CDRs<0.5 in both eyes as determined by the examining physician.
An attempt was made whenever possible to group match (within 5 years) the age of the controls to that of the cases with the balance between groups monitored on a weekly basis, and adjustments made as needed. Subjects were not matched for sex or any other demographic parameters. Enrollment was performed at the time of scheduled ophthalmic examination (either initial or follow-up). Eligible patients were provided information describing the study and were enrolled if they agreed to participate and signed the informed consent forms.
Data collection occurred during that same visit. After informed consent was obtained, a full clinical examination which included gonioscopy and dilated fundus examination was performed on all subjects (cases and controls) by the examining physician. Data were collected by trained study personnel utilizing standard protocols and study forms. In addition, glaucoma cases were subjected to automated visual field examination (HVF) and optic nerve head photography if they have not had such a test within 6 months of the time of enrollment. Following the clinical examination, subjects (both cases and controls) were provided with 30cc of Scope ™ mouthwash solution and asked to take the mouthwash into their mouth, swish for 30 seconds and spit in a sterile cup. All mouthwash specimens collected were transferred to 15cc vials by study personnel, anonymized and frozen upon arrival to the lab (within a few hours from the time of collection). Vials were frozen at −80°C and maintained at that temperature until they were transported on dry ice to the laboratory of one of the co-authors (A.G) for further analysis. There they were subjected to determination of bacterial and total DNA and genomic pyrosequencing of bacterial RNA using methodology routinely employed by A.G.
In addition, subjects were asked to complete a self-administered questionnaire to collect information about their dental health (e.g. number of natural teeth, signs of periodontal or gum disease etc. see Supplemental Material) and specific environmental exposures (e.g. cigarette smoking and alcohol use,). Questionnaire responses were reviewed by a trained member of the research team and any missing, incomplete items or discrepancies clarified. De-identified demographic data as well as corresponding clinical data and questionnaire responses were entered into a database for statistical analysis.
Sample preparation and analysis
Total DNA from a randomly selected subset of African-American subjects (28 cases and 17 controls) was isolated at the laboratory of one of the co-authors (A.G) using a bead beater as previously described [22] quantified using a Nanodrop ND-1000 Spectrophotometer, and frozen until analysis. Total bacteria levels (copy number) were quantified using a BioRad iCycler Real-Time Detection System. 20ul reactions were run in an optical grade 96 well plate, using 1x SsoFast EvaGreen Supermix (BioRad), 1ul of undiluted template DNA, 0.4uM each of primers, Eub338F (ACT CCT ACG GGA GGC AGC AG) and Eub518R (ATT ACC GCG GCT GCT GG), and RT-PCR grade water. Negative controls (1ul of water instead of template DNA) were run for each plate, in duplicate. Standard curves were generated for each experiment as previously described [23], by simultaneously running reactions on serial dilutions of P. gingivalis genomic DNA containing 107-101 copy numbers of the 16srRNA gene, in triplicate. Standard curves were automatically generated by the iCycler software, by plotting threshold cycles (Ct) vs. standards’ copy numbers. The Ct of the samples was used to extrapolate their total bacterial copy number from the standard curves, as previously described [23]. Samples were measured twice and the copy numbers averaged. If the concentration of the sample fell outside the range of the standard curve, the reactions were repeated using 10-fold serial dilutions of the template. Bacterial load counts in cases compared with those in controls after log transformation have been reported in an earlier publication[21].
Preparation of 16S rRNA gene amplicon libraries and sequencing
Oligonucleotide primers targeting both the V1–2 and V4 hypervariable regions were designed based on previously described bacterial universal primers. The sequences were modified to reflect the composition of the oral microbiota using full-length 16S rRNA sequences [24] as previously described [25]. For amplifying the V4 region, modifications of the primers described on the RDP [26] pyrosequencing website (http://rdp.cme.msu.edu/) we used as previously described [25]. Amplification of both the V1–2 and V4 regions was performed in 50-μl reactions with 20–50ng of gDNA as previously described [25]. Negative control reactions without template were included.
Amplicons were visualized on 2% (w/v) agarose gels stained with ethidium bromide and purified using AMPure paramagnetic beads (Agencourt Bioscience Corporation, USA), according to manufacturer’s protocol, followed by concentration and size-distribution analysis using DNA 1000 chips on an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Germany). Purified amplicons were prepared for sequencing using the unidirectional amplicon library sequencing protocol with the emPCR Kit II (Roche Diagnostics, USA) followed by sequencing on a 454 Life Sciences Genome Sequencer FLX (Roche Diagnostics, USA).
Sequence processing and analysis
The sequences were parsed by barcodes using the Ribosomal Database Project’s Pipeline Initial Process. V4 amplicons were screened for the presence of both forward and reverse primers, allowing up to two mismatches for each primer, no ambiguous base calls and a 200-base minimum sequence length. V1–2 amplicons were screened with up to two mismatches of the forward primer, zero ambiguous base calls and a 200-base minimum sequence length.
BLAST searches [27] were carried out against our in-house created database of oral 16S rRNA sequences [24]. Queries that matched the closest database sequence at ≥ 98% identity over an alignment of at least 150 bp were assigned to the respective species-level taxa. The remaining unassigned sequences were clustered at 1% divergence using BLAST and a custom PHP script. Clusters with 25 or more members were identified and classified as previously described [24]. The data were normalized as percent of total for each taxon for each sample and taxa averaged for the V1 and V4 sequences.
Statistical analysis
Frequencies and distributions of possible risk factors were compared between glaucoma cases and controls, using chi square testing for comparison of categorical variables. Continuous variables were compared using the t-test or the Mann-Whitney U test depending on the type of distribution. Among glaucoma cases, linear correlations of various parameters with the mean deviation (MD) of Humphrey visual fields of the worst eye were performed to assess possible relationships between these factors and severity of the disease.
For dental history variables, responses to individual questions were analyzed separately. In addition, questions indicating gingivitis (inflammation of gums) or periodontal disease (inflammation of the periodontal tissues) were grouped and also compared across groups. Current and maximal use of alcohol was compared across the study groups using t-test. Cumulative exposure to tobacco was calculated based on current use and the number of years each subject was exposed to it. Cumulative tobacco exposure was also compared across groups using the t-test.
Factors with a difference between cases and controls that reached a significance level of p<0.15 on univariate screen, were included in multivariate analyses using a logistic regression model that also included age and gender as covariates. Potential interactions were also evaluated. Separate age-adjusted analyses were also performed among cases based on disease severity (mild to moderate: (mean deviation (MD) in the most affected eye ≥−15dB) versus severe: (MD in the most affected eye <−15dB)).
In order to determine which genera were significantly different in amount between cases and controls, we compared the amount of each genus in a subset of POAG case and control samples using t-tests. Only genera with at least 3% average contribution to the total bacterial DNA within the samples were included in this analysis.
For all comparisons a p value of less than 0.05 was considered significant. The NCSS (Kaysville, UT) statistical package was used for all statistical analyses.
RESULTS
Study population
119 cases and 78 controls were enrolled in this study. Of these, overall 74.6% (147/197) of the subjects (79.8% (95/119) cases and 66.7% (52/78) controls) enrolled were African-Americans (AA) with 15.7% Hispanic, 6.1% White and 1.5% Asian. Because of the high proportion of AAs in this study, and the limited ability to draw inferences regarding associated factors in other racial groups, as well as the known high incidence of POAG among AAs, all results reported in this manuscript were limited to AAs (95 cases and 52 controls). However, results based on the full study group were similar to those reported here (data not shown).
The AA case and control groups were similar with respect to age. Mean (± SD) age was 62.2 ± 11.4 years for cases and 60.9 ± 9.7 years for controls (p>0.48, t-test). Overall, 44.9% (66/147) of the AA subjects were male and 55.1% (81/147) female. However, 51.6% (49/95) of cases vs 32.7% (17/52) of controls were male (p<0.04, Fisher’s exact test) (Table 1). No other factors evaluated, differed between the two groups (p>0.05 for all comparisons - see Table 1).
Table 1.
Demographics and other characteristics of African- American POAG Cases and Controls
| POAG Cases | Controls | p-value | |
|---|---|---|---|
| Number of participants (N (%)) | 95 | 52 | |
| Gender: | |||
| Males (N (%)) | 49 (51.57) | 17 (32.69) | 0.037 |
| Age, | |||
| years (mean (SD)) | 62.24 (11.39) | 60.92 (9.71) | NS |
| range | 40–87 | 40–84 | NS |
| Hypertension (N (%)) | 54 (62.75) | 32 (61.36) | NS |
| Diabetes (Type 1 and Type 2) (N (%)) | 36 (40.91) | 17 (33.33) | NS |
| Hypercholesterolemia (N (%)) | 22 (25.29) | 14 (27.45) | NS |
| Users of Statins (N (%)) | 26 (30.23) | 16 (32.65) | NS |
| Self-reported current alcohol consumption (ounces/wk) (mean +/− (SD)) | 0.42 (1.31) | 0.59 (1.52) | NS |
| Self-reported maximal alcohol consumption (ounces/wk) (mean +/− (SD)) | 2.90 (6.33) | 2.43 (3.32) | NS |
| Self-reported smoking exposure (Pack-years) (mean +/− (SD)) | 5.28 (12.84) | 5.62 (10.15) | NS |
| Average CCT (mean +/− (SD)) | 539.76 (40.72) | 543.27 (38.50) | NS |
T-test for the continuous demographic variables (age, CCT) (p<0.05 considered significant).
Fisher’s exact test for categorical demographic variables (gender, hypertension, diabetes, smoking status, etc.) (p<0.05 considered significant). CCT: central corneal thickness.
Cigarette pack-years calculated based on current consumption
NS: non-significant (p>0.05)
Dental health
Self-reported dental health parameters are summarized in Table 2. Although the mean (±SD) total number of natural teeth was lower in cases compared to controls (18.0 (±11.12) and 20.7 (±9.44), respectively), the difference did not reach statistical significance (p>0.14, t-test). No other dental health parameters evaluated (including gingivitis and periodontal disease, analyzed individually and as a group) showed a statistically significant difference between study groups.
Table 2.
Dental health factors among African-American POAG Cases and Controls
| Factors studied | POAG Cases (N=95) | Control (N=52) | p-value |
|---|---|---|---|
| Number of natural teeth (mean ± SD) a | 18.03 (11.12) | 20.73 (9.45) | 0.14 |
| Number of cavities (mean ± SD) | 0.17 (0.58) | 0.20 (0.66) | 0.81 |
| Number of fillings (mean ± SD) | 2.46 (3.50) | 2.88 (3.68) | 0.49 |
| Edentulism (%) b | 14.77 | 5.77 | 0.18 |
| Subjects with at least one root canal therapy (%) | 18.95 | 30.77 | 0.15 |
| Number of teeth replaced with removable dentures (mean ± SD) | 7.61 (11.42) | 6.08 (9.95) | 0.42 |
| Number of teeth replaced with implants (mean ± SD) | 0.86 (4.70) | 0.10 (0.45) | 0.24 |
| Number of teeth not replaced (mean ± SD) | 4.41 (6.17) | 3.83 (4.59) | 0.55 |
| Feeling that the teeth are loose or wobbly (%) | 4.21 | 5.77 | 0.7 |
| Periodontal or gum disease (%) | 13.04 | 13.46 | 1.0 |
| Periodontal bone loss (%) | 10.53 | 9.62 | 1.0 |
| Need of periodontal/gum treatment (%) | 22.11 | 23.08 | 1.0 |
| Had gum treatment (%) | 20.0 | 19.23 | 1.0 |
| Receding Gums (%) | 11.58 | 13.46 | 0.8 |
| Gum bleeding (often) (%) | 13.68 | 13.46 | 1.0 |
| Gum bleeding while brushing (%) | 27.37 | 30.77 | 0.71 |
| A positive response to at least one of the above eight factors (indicating periodontal or gum disease) (%) | 48.42 | 46.15 | 0.86 |
T-test for the continuous demographic variables (number of natural teeth, etc) (p<0.05 considered significant).
Fisher’s exact test for categorical demographic variables (edentulism, etc) (p<0.05 considered significant).
Of all factors evaluated, only number of natural teeth met the univariate screening criterion (p<0.15) for inclusion in the logistic regression model, that also included age and gender as covariates and all two way interactions (i.e. between age and number of teeth, gender and number of teeth and age and gender).
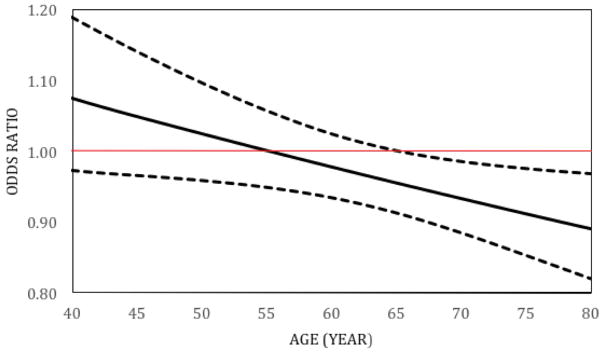
Based on this model, the number of natural teeth remained associated with POAG at older (≥ 65 years), but not younger ages (<65 years), with a significant interaction between number of natural teeth and age (p<0.02) (Table 3 and Figure 1). Thus, beginning at age 65 years, having more natural teeth was associated with a lower likelihood of having POAG, with the odds ratios decreasing with increasing age. The odds ratios (OR (95% CI)) decreased from 0.93 (0.88–0.99) at age 70 to OR= 0.87 (0.79–0.96) at age 85. For example, at age 70 with each additional tooth, the odds of having POAG is decreased by 7% and at age 85, the odds of having POAG is decreased by 13%. No other associations (including gender) or interactions were identified (p>0.20 for all factors and interactions evaluated).
Table 3.
Relationship between Number of natural teeth and POAG at different ages (N= 95 Cases and 52 Controls)
| Number of natural teeth | Adjusted Odds Ratio1 | 95% CI | p value | |
|---|---|---|---|---|
| Lower | Upper | |||
| at age 40 | 1.07 | 0.97 | 1.19 | 0.17 |
| at age 45 | 1.05 | 0.96 | 1.14 | 0.26 |
| at age 50 | 1.02 | 0.96 | 1.10 | 0.48 |
| at age 55 | 1.00 | 0.95 | 1.06 | 0.98 |
| at age 60 | 0.98 | 0.93 | 1.02 | 0.34 |
| at age 65 | 0.96 | 0.91 | 1.00 | 0.05 |
| at age 70 | 0.93 | 0.88 | 0.99 | 0.01 |
| at age 75 | 0.91 | 0.85 | 0.98 | 0.007 |
| at age 80 | 0.89 | 0.82 | 0.97 | 0.007 |
| at age 85 | 0.87 | 0.79 | 0.96 | 0.007 |
Based on a logistic regression model that includes age (as a continuous variable), gender, number of teeth as covariates and two-way interactions.
Figure 1.
Relationship between Number of natural teeth and POAG at different ages. Adjusted Odds Ratios 1 (OR) and 95% Confidence Intervals (N= 95 Cases and 52 Controls)
1Based on a logistic regression model that includes age (as a continuous variable), gender, number of natural teeth as covariates and two-way interactions.
The solid black line represents the OR’s for number of natural teeth as a risk factor for POAG at different ages. The dashed curves are 95% confidence intervals for the odds ratios. The red line is at OR=1, i.e, no association. When the upper bound of the 95% CI falls below an OR of 1 (i.e., when age is 65.1 years or older), the ORs for the number of natural teeth predicting POAG are considered to be statistically significant.
In an analysis based on glaucoma cases only, the number of teeth did not correlate with disease severity (p>0.05, linear regression). In addition the number of teeth did not correlate with the number of glaucoma medications, age, gender or their first order interactions (p>0.12 for all, GLM ANOVA). An additional exploratory analysis divided POAG cases into those with either severe disease (n=44) or “mild to moderate” glaucoma (n=51) and evaluated potential associated factors using logistic regression with age as a covariate. Participants with “mild to moderate” glaucoma were more likely to report “gum bleeding while brushing teeth” and “responding positively to at least one question suggestive of periodontal disease” than those with “severe” disease (adjusted odds ratios (95% CI): 3.77 (1.33–10.67) ; p=0.01 and 2.86 (CI 1.22–6.68 ); p=0.02, respectively.
Bacterial analysis of oral samples
A subset of 28 cases and 17 controls were randomly selected from the larger cohort of African-American participants for an exploratory analysis using the oral washout samples for bacterial DNA analysis. Of these samples, we failed to obtain a reliable measurement of the amounts of bacterial DNA in one case, which was subsequently removed from further analysis.
No significant differences in age, number of teeth or sex (p>0.05, t-tests and Fisher’s exact test) were observed between patients included in this subgroup analysis compared to those not included. There were also no significant age or sex differences (p>0.05, t-test and Fisher’s exact test respectively) between cases and controls in this subsample.
We have previously reported [21] that the total amount of normalized oral bacterial loads (NOBL) was significantly higher among cases compared to that of controls in a larger cohort of subjects. For the particular subset presented here, NOBL of cases were similarly higher than that of controls although the difference did not reach statistical significance (4.79 vs 4.52 log copies 16S/μg total DNA, p<0.12 t-test). Here we provide a more detailed analysis of the data obtained from the metagenomic analysis.
Each case and control saliva sample was evaluated for the amount (log 16S RNA copies) of oral bacterial load, and presence of particular bacterial genera and individual bacterial species within each genus. The prevalence of each particular bacterial genus (as percentage of subjects with a positively identified genus within the total number of subjects in a group) among cases and controls, was similar (p>0.05, Chi-square test).
The seven genera that contributed the most to the total amount of bacterial load of cases and controls are shown in Table 3, with each contributing on average at least 3% to the total oral bacterial load in both groups. Among these genera, the amounts of Streptococci were significantly higher among cases (45.5%) compared to controls (38.8%) (p<0.023, t-test)(Table 4).
Table 4.
Percent contribution of various bacterial genera1 to the oral bacterial loads of a subset of AA POAG cases and controls.
| Bacterial Genus | Cases (N=27) % contribution |
Controls (N=17) % contribution |
Significance level (p value)2 |
|---|---|---|---|
|
| |||
| Streptococcus | 45.5 | 38.8 | 0.0233 |
| Rothia | 11.2 | 12.3 | NS |
| Prevotella | 11.4 | 11.8 | NS |
| Veillonella | 6.8 | 5.8 | NS |
| Porphyromonas | 2.9 | 3.8 | NS |
| Actinomyces | 3.3 | 3.7 | NS |
| Neisseria | 3.1 | 3.0 | NS |
Only genera with at least 3% average contribution are listed. Normalized amounts of the various genera were similar among cases and controls with the exception of Streptococci. NS: not statistically significant (p>0.05).
p values are based on t-tests comparing the log of 16S RNA copies in 27 cases and 17 controls
Test statistically significant after Benjamini-Hockberg correction with False Discovery Rate FDR=0.2.
At the species level, while present in 100% of cases and controls, the amounts of Streptococcus mitis were higher in cases compared to controls (p<0.027, t-test) and accounted for ~26% of all bacteria in cases and for ~17.6% of the bacteria in controls.
In addition, although the Neisseria genus was present in 80% of case samples and 65% of the control samples (p>0.05), amounts of the Neisseria meningitidis polysaccharea species were higher among cases compared to controls (p<0.005, Mann-Witney U test as amounts did not follow the normal distribution) and this species was one of the species that was more prevalent among cases compared with controls (detected in 53.6% of cases and 5.9% of controls, p=0.0012, Fisher’s exact test).
Two additional species (Actinomyces graevenitzii, Actinomyces massiliensis) also differed between cases and controls, with lower frequency of Actinomyces graevenitzii (52.9% vs. 89.3%) and higher frequency of Actinomyces massiliensis (52.9% vs. 14.3%) in the cases, p=0.0109 and p=0.0083, respectively) although the % amounts that these species represented in samples of both groups was rather low (ranging between ~0.08% and ~1%).
In an analysis based on glaucoma cases only, the total amount of oral bacterial load was not correlated with the mean deviation (MD) of the worst eye of individual glaucoma cases (p>0.05). In contrast, correlations of the MD with the amounts of particular bacterial genera was stronger (R2 up to 0.28, see table 5).
Table 5.
Associations of specific bacterial genera with degree of visual field damage (measured as mean deviation in the worst eye) in a subset of POAG cases (n= 27).
| Bacterial genus | Correlation coefficient (R2)1,2 | Significance level (p value)3 |
|---|---|---|
|
| ||
| Actinobaculum | 0.01 | NS |
| Actinomyces | 0.00 | NS |
| Bacteroides | 0.06 | NS |
| Campylobacter | 0.02 | NS |
| Catonella | 0.23 | 0.011 |
| Enterococcus | 0.02 | NS |
| Haemophilus | 0.01 | NS |
| Mycoplasma | 0.14 | NS |
| Neisseria | 0.01 | NS |
| Parvimonas | 0.22 | 0.015 |
| Porphyromonas | 0.02 | NS |
| Propionibacterium | 0.00 | NS |
| Pseudomonas | 0.01 | NS |
| Rothia | 0.07 | NS |
| Staphylococcus | 0.00 | NS |
| Streptococcus | 0.02 | NS |
| Synergistes | 0.18 | 0.028 |
| Treponema | 0.16 | 0.036 |
| unclassified Clostridiales C | 0.28 | 0.005 |
Correlations and associated p values are based on linear regression analysis
Statistically significant correlations are highlighted with R2 ranging from 0.16 to 0.28 indicating week correlation.
NS: not statistically significant (p>0.05).
DISCUSSION
This pilot case-control study explored a possible association between dental health and POAG in a sample of 95 AA POAG cases and 52 controls without glaucoma and found a protective association between POAG and having more natural teeth, at older, but not younger ages, in a multivariate analysis adjusting for gender and age. The protective effect increased with increasing age (ORs decreasing from 0.96 at age 65 years to 0.87 at age 85 years). This observation, suggests that there is an inverse relationship between having more teeth and the odds of having POAG, that increases with increasing age, providing support for the hypothesis that chronic dental infections may affect glaucomatous neurodegeneration [21]. In addition, results of an exploratory analysis in a small subset of cases and controls, showed increased amounts of Streptococci in the oral cavity of cases compared to that of controls lending support to the possibility that specific commensal bacteria may be responsible for this effect.
The study population was recruited from an urban population where African-Americans are over-represented and where glaucoma is highly prevalent. In fact, a large percentage of the population from which this study sample is drawn, is of Afro-Caribbean descent where glaucoma is present in ~8% of the population [28]. As such, the results may or may not be applicable to other populations with different ethnic and race composition.
For this case-control study we elected to impose only optic nerve (and IOP) criteria for inclusion in the control group. Visual fields were not obtained from controls. However, that is unlikely to have resulted in false negatives as visual field changes typically occur in glaucoma when a significant amount of nerve fibers have already been lost and thus some cupping has already developed [29]. In contrast, we imposed rather strict criteria for inclusion in the case group. These criteria included both typical optic nerve head as well as characteristic visual field changes. As a result, most of the patients with glaucoma enrolled in the study had what would be considered moderate to severe disease. That would of course imply that they had the disease for a considerable amount of time before being enrolled. However, the duration of glaucoma was not recorded and in any case the time from diagnosis is not necessarily reflective of the time of initiation of glaucoma pathology. As such, the findings presented here cannot be considered to indicate a causative relationship. They are however raising significant questions and may allow us to explore further specific environmental factors that may be contributing to glaucoma potentially through modifying dental health.
Although cases and controls were similar in age, the two groups differed in their gender composition with more females in the control group compared to the case group. Although the prevalence of POAG has not been shown to differ between males and females in large epidemiologic studies [6–8, 30], it is possible that the gender imbalance in the current study could have influenced the results observed. However, gender or interactions with gender were not found to be associated with POAG in the multivariable analysis, suggesting that this imbalance did not influence the study findings.
Other than the gender differences in cases and controls, the two study groups had similar frequencies of other potentially confounding factors like the presence of diabetes, hypertension and hypercholesterolemia. It is interesting that corneal thickness was not different between the two groups. Corneal thickness has been identified as one of the strongest risk factors in the development of glaucoma [5, 31] and thin corneas have been reported to be more prevalent in AAs with the disease [9, 32]. In the current study, the corneal thickness did not differ between cases and controls as has also been reported by others [33]. However, corneal thicknesses appear to be slightly higher than the values previously reported for AAs [32, 33]. This may be the result of population differences or a result of the fact that we report average CCTs of the two eyes of each subject.
As with prior studies [34], tobacco consumption was not associated with the presence of glaucoma. In addition tobacco cumulative exposure was not more prevalent in cases than controls and did not correlate with the severity of the disease in patients with glaucoma. Similarly, alcohol consumption (either current or maximal) was not associated with the disease status. Thus, despite the suggestion that excessive tobacco and alcohol consumption can affect the optic nerve causing tobacco-alcohol optic neuropathy [35], the range of exposure encountered in this study does not seem to significantly affect the risk of having glaucoma or having more severe disease.
To investigate the relationship of dental health with glaucoma we used a comprehensive questionnaire. The questions included were assembled from previously published reports that have investigated their validity in measuring a number of parameters that indicate dental health [36]. The questions (individually or as a group) have been shown to have high sensitivity and moderate specificity for periodontal disease and some for gingivitis [37]. These conditions have been previously associated with other health conditions including atherosclerosis, peripheral arterial disease [38] and obesity [39].
In this study, it is intriguing that the average number of natural teeth in non-glaucomatous controls was higher than that in POAG cases (although this difference did not reach statistical significance). The number of natural teeth (and the presence of edentulism) have been previously used as crude measures of dental health [40, 41]. Thus, differences in the amount of teeth of cases compared to controls suggest that glaucoma cases have poorer dental health. In case-control studies causality cannot be ascribed. It can for example be argued that glaucoma leads to poorer dental health because of poorer vision or changes in the oral flora secondary to use of ocular medications. Both of these explanations, however, would imply that as glaucoma gets worse (and vision declines or ocular medication use increases) dental health would deteriorate further. The lack of an association between the number of natural teeth and the disease severity argues against poorer dental health being the result of glaucoma. In addition the number of natural teeth was not associated with the number of glaucoma medications used within cases suggesting that glaucoma medication use does not contribute to tooth loss (although it may be affecting the oral microbiome).
Alternatively, in susceptible individuals glaucoma may be triggered by poor dental health. We have previously shown that peripheral infections can lead to microglial activation that can exacerbate glaucomatous neurodegeneration [21]. Infections that result in tooth loss are often associated with significant periodontal disease. Interestingly, the self-reported measures of periodontal disease or gingivitis were not associated with POAG status suggesting that remotely past dental infections may be more important than recent infections in glaucoma development.
Although, from a practical standpoint these findings cannot be used as a rationale for treatment of periodontal infections in patients with glaucoma, from a public health perspective it may be worthwhile exploring the possibility of glaucoma prevention in high risk populations by improving dental care.
It is intriguing that contrary to expectation, patients with milder POAG had slightly (but statistically) increased signs of periodontal disease compared to those with more severe disease. This would suggest that periodontal infections temporally precede the development of severe glaucomatous damage and may thus be contributing to ongoing glaucomatous neurodegeneration. If so, improved dental care may become relevant even to patients with established glaucoma. In support of such a possibility it was recently reported (Pasquale et al, American Glaucoma Society 2016 meeting, Ft Lauderdale FL) that tooth loss was higher in the two year period preceding diagnosis of glaucoma among participants in the Health Professionals Study that went on to develop the disease.
In an exploratory analysis of the bacterial composition of a subsample of mouthwash samples from cases and controls we attempted to identify possible commensal bacteria that may be associated with the presence of glaucoma. In previous work we have shown that oral bacterial loads were higher among patients with glaucoma than controls [21]. Here we attempted to determine whether specific bacterial genera or individual bacterial species account for these differences. Although we appreciate that some of these findings may be false positives due to multiple comparisons or chance associations and need confirmation in other studies, we report them here because they seem to follow an interesting pattern and raise the possibility of a connection of certain bacteria with neurodegeneration in glaucoma.
The percentage of cases and controls that carried individual bacterial genera was not different. However, the amount of Streptococci was significantly higher in cases than in controls. Most of this difference was attributable to the amounts of Streptococcus mitis (which includes Streptococcus pneumoniae, Streptococcus infantis and Streptococcus oralis) which non-the-less appeared to be present in all cases and all controls. In addition, amounts of Neisseria meningitidis polysaccharea were higher and the same species was more prevalent among cases than controls even though bacteria of the Neisseria genus were present in a similar percentage of the samples from both cases and controls
It is interesting that of the three major bacterial meningitis pathogenic species (Streptococcus pneumoniae, Neisseria meningitidis, and Hemophilus influenza) two seem to be potentially associated with glaucoma in this cohort. Group B as well as Group A Streptococci have been linked to neurodegeneration as well as specific neurologic conditions [42–44]. Neurotoxicity of Group B Streptococci and S. pneumoniae seem to be mediated by nitric oxide production through effects on microglia and astrocytes [45]. Although it is unclear from the present work whether bacteria may have a local effect at the optic nerve head we have previously shown that lipopolysaccharide (LPS) treatment induces specific changes in microglia and the TLR4 pathway in the optic nerve and retina [21]. The results of that work suggest that localization of bacteria in these CNS structures is not necessary (as for example has been observed in atheromatous plaques) to induce inflammatory responses. In fact such localization has not been reported and is rather unlikely. Given the increased permeability of optic nerve head capillaries [46] it is likely that bacterial products (rather than intact bacteria) can come in contact with resident microglia in this region to initiate or enhance a local inflammatory response that can lead to axonal neurodegeneration.
It is unclear whether differences in prevalence of rarer species identified in this cohort represent real differences between the glaucoma cases and non-glaucomatous control populations. Given the low prevalence to these species these results should be viewed with caution until confirmed in larger samples.
Finally, it is important to point out that the total amount of oral bacterial loads were not correlated with disease severity among cases, and while a number of genera did correlate with disease severity streptococcal loads did not correlate with it. This finding implies that a threshold of Streptococci may be necessary to prime glaucomatous neurodegeneration, but that it may not be needed to sustain this process.
As a pilot case control study with a small sample size and limited resources, this study has a number of potential limitations that should be considered. Groups were not equal in size and were not matched for gender and the resulting gender imbalance between cases and controls, although addressed in multivariate analyses, may have influenced the final results. In addition, the analyses for this study population were limited to AA cases and controls, who have an increased prevalence of glaucoma. Thus, it is unclear whether the results would apply to other populations. In addition, the sample size of the metagenomics study is too small to allow determination of the effect of bacterial genera that account for a smaller percentage of the total bacterial population in the oral cavity. Given the above and other limitations, the results of this study should be considered preliminary in nature. However, the findings regarding number of natural teeth and POAG combined with the ocular microbiome findings are intriguing, provide support for proposed hypotheses and warrant further investigation. If an association between dental health, the oral microbiome and POAG were established, this would have potentially significant implications for the understanding and management of glaucoma and possibly other neurodegenerative disease as well as from a public health perspective. However, confirmation of these findings in other studies involving separate cohorts is first needed.
Supplementary Material
Acknowledgments
Funding/Support: R01 EY015224, Unrestricted challenge grant to the Department of Ophthalmology SUNY Downstate, Empire Clinical Research Investigator Program (ECRIP) fellowship, SUNY Eye Institute pilot grant. The sponsors and funding organizations had no role in the design or conduct of this research.
Footnotes
Financial Disclosures: No financial disclosures.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994;112(1):69–73. doi: 10.1001/archopht.1994.01090130079022. [DOI] [PubMed] [Google Scholar]
- 3.Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1640–5. doi: 10.1001/archopht.116.12.1640. [DOI] [PubMed] [Google Scholar]
- 4.Teikari JM. Genetic factors in open-angle (simple and capsular) glaucoma. A population-based twin study. Acta Ophthalmol (Copenh) 1987;65(6):715–20. doi: 10.1111/j.1755-3768.1987.tb07069.x. [DOI] [PubMed] [Google Scholar]
- 5.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–20. doi: 10.1001/archopht.120.6.714. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Varma R, Wu S, et al. Baseline risk factors that predict the development of open-angle glaucoma in a population: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119(11):2245–53. doi: 10.1016/j.ophtha.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. II. Association of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. Am J Epidemiol. 1977;106(1):33–41. doi: 10.1093/oxfordjournals.aje.a112429. [DOI] [PubMed] [Google Scholar]
- 8.Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99(10):1499–504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B, Group BES. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115(1):85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Alward WL. The genetics of open-angle glaucoma: the story of GLC1A and myocilin. Eye (Lond) 2000;14(Pt 3B):429–36. doi: 10.1038/eye.2000.127. [DOI] [PubMed] [Google Scholar]
- 11.Blue Mountains Eye S, Wellcome Trust Case Control C. Genome-wide association study of intraocular pressure identifies the GLCCI1/ICA1 region as a glaucoma susceptibility locus. Hum Mol Genet. 2013;22(22):4653–60. doi: 10.1093/hmg/ddt293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, Kim K, Heo DW, et al. Expression-associated polymorphisms of CAV1-CAV2 affect intraocular pressure and high-tension glaucoma risk. Mol Vis. 2015;21:548–54. [PMC free article] [PubMed] [Google Scholar]
- 13.Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res. 2009;88(4):837–44. doi: 10.1016/j.exer.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacca SC, Bolognesi C, Battistella A, Bagnis A, Izzotti A. Gene-environment interactions in ocular diseases. Mutat Res. 2009;667(1–2):98–117. doi: 10.1016/j.mrfmmm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Zhang X, Cai S, Ma J, Liu X, Wang N. Correlated or not: Glaucoma prevalence and modern industrialization. Med Hypotheses. 2011;76(2):220–4. doi: 10.1016/j.mehy.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Newman-Casey PA, Talwar N, Nan B, Musch DC, Pasquale LR, Stein JD. The potential association between postmenopausal hormone use and primary open-angle glaucoma. JAMA Ophthalmol. 2014;132(3):298–303. doi: 10.1001/jamaophthalmol.2013.7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquale LR, Kang JH. Female reproductive factors and primary open-angle glaucoma in the Nurses’ Health Study. Eye (Lond) 2011;25(5):633–41. doi: 10.1038/eye.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewundara S, Pasquale LR. Exfoliation syndrome: a disease with an environmental component. Curr Opin Ophthalmol. 2015;26(2):78–81. doi: 10.1097/ICU.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein JD, Pasquale LR, Talwar N, et al. Geographic and climatic factors associated with exfoliation syndrome. Arch Ophthalmol. 2011;129(8):1053–60. doi: 10.1001/archophthalmol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaik MM, Ahmad S, Gan SH, et al. How do periodontal infections affect the onset and progression of Alzheimer’s disease? CNS Neurol Disord Drug Targets. 2014;13(3):460–6. doi: 10.2174/18715273113126660152. [DOI] [PubMed] [Google Scholar]
- 21.Astafurov K, Elhawy E, Ren L, et al. Oral microbiome link to neurodegeneration in glaucoma. PLoS One. 2014;9(9):e104416. doi: 10.1371/journal.pone.0104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross EL, Leys EJ, Gasparovich SR, et al. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol. 2010;48(11):4121–8. doi: 10.1128/JCM.01232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons SR, Griffen AL, Leys EJ. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J Clin Microbiol. 2000;38(6):2362–5. doi: 10.1128/jcm.38.6.2362-2365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffen AL, Beall CJ, Firestone ND, et al. CORE: a phylogenetically-curated 16S rDNA database of the core oral microbiome. PLoS One. 2011;6(4):e19051. doi: 10.1371/journal.pone.0019051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6(6):1176–85. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole JR, Wang Q, Cardenas E, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37(Database issue):D141–5. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Leske MC, Troutman HT, Connell A. Glaucoma in Barbados. Arch Ophthalmol. 1989;107(2):169. doi: 10.1001/archopht.1989.01070010175013. [DOI] [PubMed] [Google Scholar]
- 29.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109(1):77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 30.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266(3):369–74. [PubMed] [Google Scholar]
- 31.Nemesure B, Wu SY, Hennis A, Leske MC Barbados Eye Study G. Corneal thickness and intraocular pressure in the Barbados eye studies. Arch Ophthalmol. 2003;121(2):240–4. doi: 10.1001/archopht.121.2.240. [DOI] [PubMed] [Google Scholar]
- 32.Aghaian E, Choe JE, Lin S, Stamper RL. Central corneal thickness of Caucasians, Chinese, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmology. 2004;111(12):2211–9. doi: 10.1016/j.ophtha.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Mercieca K, Odogu V, Fiebai B, Arowolo O, Chukwuka F. Comparing central corneal thickness in a sub-Saharan cohort to African Americans and Afro-Caribbeans. Cornea. 2007;26(5):557–60. doi: 10.1097/ICO.0b013e3180415d90. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Kahende J, Fan AZ, et al. Smoking and visual impairment among older adults with age-related eye diseases. Prev Chronic Dis. 2011;8(4):A84. [PMC free article] [PubMed] [Google Scholar]
- 35.Chiotoroiu SM, Noaghi M, Stefaniu GI, Secureanu FA, Purcarea VL, Zemba M. Tobacco-alcohol optic neuropathy--clinical challenges in diagnosis. J Med Life. 2014;7(4):472–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Pitiphat W, Garcia RI, Douglass CW, Joshipura KJ. Validation of self-reported oral health measures. J Public Health Dent. 2002;62(2):122–8. doi: 10.1111/j.1752-7325.2002.tb03432.x. [DOI] [PubMed] [Google Scholar]
- 37.Blicher B, Joshipura K, Eke P. Validation of self-reported periodontal disease: a systematic review. J Dent Res. 2005;84(10):881–90. doi: 10.1177/154405910508401003. [DOI] [PubMed] [Google Scholar]
- 38.Zoellner H. Dental infection and vascular disease. Semin Thromb Hemost. 2011;37(3):181–92. doi: 10.1055/s-0031-1273082. [DOI] [PubMed] [Google Scholar]
- 39.Saito T, Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol 2000. 2007;43:254–66. doi: 10.1111/j.1600-0757.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 40.Unell L, Soderfeldt B, Halling A, Paulander J, Birkhed D. Oral disease, impairment, and illness: congruence between clinical and questionnaire findings. Acta Odontol Scand. 1997;55(2):127–32. doi: 10.3109/00016359709115404. [DOI] [PubMed] [Google Scholar]
- 41.Ramos RQ, Bastos JL, Peres MA. Diagnostic validity of self-reported oral health outcomes in population surveys: literature review. Rev Bras Epidemiol. 2013;16(3):716–28. doi: 10.1590/s1415-790x2013000300015. [DOI] [PubMed] [Google Scholar]
- 42.Dale RC. Post-streptococcal autoimmune disorders of the central nervous system. Dev Med Child Neurol. 2005;47(11):785–91. doi: 10.1017/S0012162205001647. [DOI] [PubMed] [Google Scholar]
- 43.Lehnardt S, Henneke P, Lien E, et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177(1):583–92. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 44.Libbey JE, Fujinami RS. Role for antibodies in altering behavior and movement. Autism Res. 2010;3(4):147–52. doi: 10.1002/aur.144. [DOI] [PubMed] [Google Scholar]
- 45.Kim YS, Tauber MG. Neurotoxicity of glia activated by gram-positive bacterial products depends on nitric oxide production. Infect Immun. 1996;64(8):3148–53. doi: 10.1128/iai.64.8.3148-3153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofman P, Hoyng P, vanderWerf F, Vrensen GF, Schlingemann RO. Lack of blood-brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci. 2001;42(5):895–901. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.