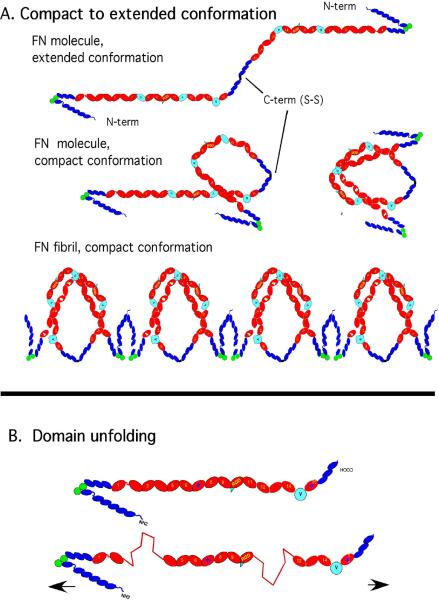
Fig. 2.
Two models for the mechanism of stretching FN matrix fibrils. The top model is based on the compact-to-extended conformational change (modified from [17]). FN dimers in physiological solution fold into a compact pretzel-like shape. This may involve domains III-2-3 of one subunit forming electrostatic bonds to III-12-14 of the other subunit [8]; the N-terminal FNI domains probably also play a role [1]. If molecules are connected by their N-terminal FNI domains, initially in the compact conformation, extending them fully would generate a 3.5-fold extension. The bottom model shows the proposal for tension progressively unfolding FNIII domains and extending them to produce the stretch.