Abstract
Agrammatic aphasia can be observed in neurodegenerative disorders and has been traditionally linked with damage to Broca’s area, although there have been disagreements concerning whether damage to Broca’s area is necessary or sufficient for the development of agrammatism. We aimed to investigate the neuroanatomical correlates of the emergence of agrammatic aphasia utilizing a unique cohort of patients with primary progressive apraxia of speech (PPAOS) that did not have agrammatism at baseline but developed agrammatic aphasia over time. Twenty PPAOS patients were recruited and underwent detailed speech/language assessments and 3T MRI at two visits, approximately two years apart. None of the patients showed evidence of agrammatism in writing or speech at baseline. Eight patients developed aphasia at follow-up (progressors) and 12 did not (non-progressors). Tensor-based morphometry utilizing symmetric normalization was used to assess patterns of grey matter atrophy and voxel-based morphometry was used to assess patterns of grey matter loss at baseline. The progressors were younger at onset and more likely to show distorted sound substitutions or additions compared to non-progressors. Both groups showed change over time in premotor and motor cortices, posterior frontal lobe, basal ganglia, thalamus and midbrain, but the progressors showed greater rates of atrophy in left pars triangularis, thalamus and putamen compared to non-progressors. The progressors also showed greater grey matter loss in pars triangularis and putamen at baseline. This cohort has provided a unique opportunity to assess the anatomical changes that accompany the development of agrammatic aphasia. The results suggest that damage to a network of regions including Broca’s area, thalamus and basal ganglia are responsible for the development of agrammatic aphasia. Clinical and neuroimaging abnormalities were also present before the onset of agrammatism that could help improve prognosis in these subjects.
Keywords: Agrammatism, aphasia, apraxia of speech, Broca’s area, magnetic resonance imaging
1. INTRODUCTION
Agrammatic aphasia can be observed in neurodegenerative disorders and is characterized by grammatical errors in speech or writing and impairments in comprehending syntactically complex sentences (Thompson & Mack, 2014). Early stroke studies associated the presence of agrammatic aphasia with damage to Broca’s area, located in the left inferior frontal lobe (Broca, 1865; Geschwind, 1970), although there have been disagreements in the literature concerning whether damage to Broca’s area is necessary or sufficient for the development of agrammatic aphasia (Fridriksson, Fillmore, Guo, & Rorden, 2015; Marie, 1906). Patients have been reported with agrammatic aphasia that do not have strokes affecting Broca’s area, and, conversely, patients have been reported with strokes in Broca’s area that do not have agrammatism (Fridriksson et al., 2015; Marie, 1906; Yang, Zhao, Wang, Chen, & Zhang, 2008). Several stroke studies have suggested that damage to other structures, including the insula, parietal lobe, superior temporal lobe, thalamus and basal ganglia are also important in the development of agrammatism (Dronkers, Plaisant, Iba-Zizen, & Cabanis, 2007; Jodzio, Gasecki, Drumm, Lass, & Nyka, 2003; Mohr et al., 1978; Yang et al., 2008).
Structural neuroimaging has also been used to try to pin-point the anatomic correlate of agrammatic aphasia in neurodegenerative disorders. These studies have typically assessed patterns of atrophy in groups that have the agrammatic variant of primary progressive aphasia (Gorno-Tempini et al., 2004; Grossman et al., 2013; Josephs et al., 2013; Josephs et al., 2006; Rohrer et al., 2009), and a couple of studies have examined correlations between clinical measures of agrammatic aphasia severity and grey matter volume (Amici et al., 2007; Whitwell, Duffy, Strand, Xia, et al., 2013). The latter approach identified correlates in Broca’s area, but also in the middle and superior frontal gyri and even temporal regions (Amici et al., 2007; Whitwell, Duffy, Strand, Xia, et al., 2013). The weakness of these approaches is that patients with agrammatic aphasia often have other clinical features, such as apraxia of speech (Josephs et al., 2006) or parkinsonism (Graff-Radford, Duffy, Strand, & Josephs, 2012; Josephs et al., 2006; Kertesz, McMonagle, Blair, Davidson, & Munoz, 2005), and hence it can be difficult to disentangle the possible causal relationships between anatomy and these different clinical features. In addition, the patients in these studies already had well established agrammatic aphasia and so it has not been possible to assess the changes in the brain that occur before symptom onset or at the time of development of agrammatic aphasia.
Primary progressive apraxia of speech (PPAOS) is a neurodegenerative motor speech disorder characterized by isolated apraxia of speech (J.R Duffy, 2013), in the absence of agrammatic aphasia (Josephs et al., 2012); however, we have recently demonstrated that some patients with PPAOS develop agrammatic aphasia over time, while in others apraxia of speech remains the sole feature (Josephs et al., 2014). Thus, patients with PPAOS provide a unique opportunity to study the emergence of agrammatic aphasia. The aim of this study was, therefore, to study a cohort of PPAOS subjects that had been followed longitudinally to assess the neuroimaging changes that accompany the development of agrammatic aphasia when it occurs. A comparison of PPAOS subjects that develop agrammatic aphasia to those that do not allowed us to identify regions specifically associated with the development of aphasia, rather than general worsening of other clinical features. We have shown that patients with PPAOS demonstrate a relatively focal pattern of atrophy, with grey and white matter loss observed in the supplementary motor area and superior lateral premotor regions (Josephs et al., 2013; Josephs et al., 2012; Whitwell, Duffy, Strand, Machulda, et al., 2013). Hence, we hypothesized that the development of agrammatic aphasia in PPAOS would be associated with a spread of disease from superior premotor regions into inferior frontal regions, including Broca’s area.
2. MATERIALS AND METHODS
2.1. Subjects
A cohort of 20 subjects with PPAOS underwent two serial MRIs with an interval of approximately two years. All subjects had been recruited into a cross-sectional National Institute of Health (NIH) funded grant and had been given a diagnosis of PPAOS at their first, i.e. baseline, research visit after undergoing an extensive speech and language battery, as previously described (Josephs et al., 2012). Subjects were diagnosed with PPAOS if the dominant presenting sign was apraxia of speech and any other nonspeech neurological or aphasia characteristics were considered absent or, at most, equivocal. Dysarthria was not an exclusionary problem unless it was judged as more severe than PPAOS at initial assessment. Diagnosis was made after review of video and audio recordings and speech and language test scores by two speech-language pathologists. All subjects returned for follow-up as soon as possible as part of another NIH-funded longitudinal grant. All subjects underwent an identical neurological evaluation, speech and language examination and volumetric head MRI at both baseline and follow-up. All MRI scans were performed within two days of the neurological and speech and language examinations for every subject. The video and audio recordings and speech and language test scores from the follow-up assessments were reviewed by two speech-language pathologists in a consensus meeting to determine whether agrammatic aphasia was present. The presence of agrammatic aphasia was based on evidence of agrammatism in either written or spoken language. A designation of agrammatism was made if the speech or writing sample included two or more instances of function word omission, errors in word order, or inappropriate morphology (e.g., verb tense). Subjects who developed agrammatic aphasia often also performed abnormally on other language tasks in a manner consistent with a diagnosis of aphasia (e.g., abnormal comprehension of complex sentences, abnormal confrontation naming). No subject met consensus criteria for a diagnosis of the semantic or logopenic variants of PPA (Gorno-Tempini et al., 2011). Fifteen of the 20 subjects have been screened for mutations in the three major frontotemporal dementia-related genes (progranulin (Baker et al., 2006), microtubule associated protein tau (Hutton et al., 1998) and C9ORF72 repeat expansions (DeJesus-Hernandez et al., 2011; Renton et al., 2011)) and all were negative (Flanagan et al., 2015).
The 20 PPAOS subjects were matched 2:1 by age, gender and scan interval to 40 healthy controls. All healthy controls had been recruited into the Mayo Clinic Study of Aging (MCSA) and the controls were identified from the MCSA database. The healthy control cohort had a median (IQR) age of 71 (63.75, 76) years at baseline MRI, 45% were female, and the median MRI interval was 2.3 (1.5, 2.6) years.
2.2. Speech and language battery
The speech and language battery has been described in detail previously (Josephs et al., 2012). The battery included the Western Aphasia Battery (WAB) (Kertesz, 2007), Part 1, which provided the WAB aphasia quotient (WAB-AQ) as a measure of global language ability, and the information content, fluency, and auditory verbal comprehension subscores. The battery also included the Token Test, Part V (De Renzi & Vignolo, 1962) as a sensitive measure of verbal comprehension and, more specifically, grammatic/syntactic comprehension, the 15-item Boston Naming Test (Lansing, Ivnik, Cullum, & Randolph, 1999) as a measure of confrontation-naming ability, and a Motor Speech Disorders (MSD) scale (adapted with minimal changes from (Yorkston, Strand, Miller, Hillel, & Smith, 1993)) which rates the severity of motor speech impairment (apraxia of speech and/or dysarthria) on a 10-point scale based on the functional impact of the speech disturbance (1 = nonvocal; 5=frequent repetition required; 10 = normal speech). A 0–4 rating scale (0 = normal/no impairment; 4 = severe impairment with significantly reduced speech intelligibility) was used to index AOS severity. The rating was based on conversational and narrative speech and repetition of words and sentences. Letter (FAS) and semantic (animal) fluency scores were also obtained. The writing sample from the WAB picture description was also reviewed for the presence or absence of agrammatism. Because dysarthria was not an exclusionary criterion, its severity was rated on a 0–4 severity scale (0 = normal speech; 4 = severe dysarthria). In addition, each subject was classified with an apraxia of speech type based on the specific apraxia of speech characteristics (Josephs et al., 2013). A designation of apraxia of speech Type 1 was made if articulatory as opposed to prosodic abnormalities predominated (Josephs et al., 2013). That is, there was a predominance of one or more of the following: distorted sound substitutions or additions (often increasing in prominence with increased utterance length or syllable or word complexity); sound or syllable repetitions; false starts or attempts to correct articulatory errors. A designation of apraxia of speech Type 2 was made if syllable segmentation within multisyllabic words or across words in phrases, and lengthened intersegment durations between syllables, words or phrases, was judged to clearly dominate the speech pattern (Josephs et al., 2013). If no clear predominance of Type 1 or Type 2 features was observed, or if the patient became too severe to determine the relative predominance of Type 1 and Type 2 features, a designation of AOS-NOS (not otherwise specified) was made (Josephs et al., 2013). These classifications were made by consensus between two speech-language pathologists, with excellent agreement as previously published (Josephs et al., 2013). The distinction between apraxia of speech and dysarthria was based on the identification of features commonly associated with apraxia of speech but not dysarthria (e.g., distorted sound substitutions, articulatory groping, increased errors with increased length and complexity) and features commonly associated with dysarthria but not apraxia of speech (e.g., strained voice quality, hypernasality) (J.R Duffy, 2013).
2.3. Neurological and neuropsychological batteries
All PPAOS subjects underwent detailed neurological examination by a behavioral and movement disorders specialist (KAJ) and detailed neuropsychological testing. The neurological examination included assessing general cognitive function with the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975); executive function with the Frontal Assessment Battery (FAB) (Dubois, Slachevsky, Litvan, & Pillon, 2000); praxis with the Limb Apraxia subscale of the WAB (Kertesz, 2007); functional performance with the Clinical Dementia Rating Scale sum of boxes (CDR-SB) (Hughes, Berg, Danziger, Coben, & Martin, 1982); neuropsychiatric features with the brief questionnaire form of the Neuropsychiatric Inventory (NPI-Q) (Kaufer et al., 2000), and motor function with the Movement Disorders Society sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Parts III (Goetz et al., 2008). The neuropsychological examination included assessment of memory with the Auditory Verbal Learning Test (AVLT) (Rey, 1964) delayed recall; executive function using the Delis-Kaplan Executive Function system (DKEFS) card sort(Delis, Kaplan, & Kramer, 2001); and visuoperceptual function using the Visual Object and Space Perception Battery (VOSP) incomplete letters (Warrington & James, 1991). Mayo Older American Normative Studies (MOANS) age-adjusted scaled scores (Ivnik et al., 1992) were used for the AVLT and scaled scores based on published norms were used for the DKEFS. The MOANS and DKEFS scores are constructed to have a mean of 10 and standard deviation of 3 in cognitively healthy participants.
2.4. Neuroimaging
All PPAOS and control subjects had both volumetric MRI performed at 3T using a standardized protocol. A 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) was performed at each time-point using the following parameters: TR/TE/T1, 2300/3/900 ms; flip angle 8°, 26-cm FOV; 256 × 256 in-plane matrix with a phase FOV of 0.94, voxel sizes of 1×1×1.2mm. All MPRAGE images underwent pre-processing correction for gradient non-linearity (Jovicich et al., 2006) and intensity non-uniformity using a combination of N3 (Sled, Zijdenbos, & Evans, 1998) and the SPM5 normalization (www.fil.ion.ucl.ac.uk/spm).
Longitudinal patterns of grey matter atrophy were assessed using an in-house developed version of tensor-based morphometry using symmetric normalization (TBM-SyN) (Jack et al., 2014) and Statistical Parametric Mapping (SPM5) (www.fil.ion.ucl.ac.uk/spm) software. The symmetric normalization forces the deformation between two images to be symmetric with respect to the direction of the deformation, eliminating asymmetry in the registrations and ensuring that the absolute changes from scan A to scan B are the same as from B to A (Fox, Ridgway, & Schott, 2011). First, all the repeat scans were iteratively registered to their common mean, using a nine degrees-of-freedom (9DOF) rigid-body registration. A differential-bias correction was then performed in order to balance image intensities across each scan-pair (Lewis & Fox, 2004). The symmetric normalization (SyN) algorithm (Avants, Epstein, Grossman, & Gee, 2008) was applied to each 9DOF registered and differential bias corrected scan-pair. The SyN algorithm was used to compute a nonlinear deformation required to transform the later image to the earlier image for each pair of scans, producing an image of the log of the Jacobian determinants for each deformation. The log Jacobian image for each subject was scaled by the inter-scan interval, producing annualized log Jacobian images, normalized to template space (Vemuri et al., 2008) for statistical comparison, and smoothed at 10mm full-width at half maximum. Two-sided t-tests were used to compare the annualized log Jacobians between groups including age and sex as covariates. As a secondary analysis, regression analyses were performed to assess correlations between clinical measures and rates of atrophy at the voxel-level. Results were assessed corrected for multiple comparisons using the family wise error correction at p<0.05 and uncorrected at p<0.001.
In addition, region-level rates of atrophy were calculated for each subject. For the region-level analysis the SyN deformation was calculated between each pair of scans, in both directions explicitly, saving an image of the log of the Jacobian determinants for each scan-pair which was then annualized. The SyN deformation was applied to warp the late image to the early image and the warped late image was then averaged with the early image to form a softmean image in the space of the baseline image. We then applied unified segmentation to each softmean image, and propagated automated anatomical labeling (AAL) (Tzourio-Mazoyer et al., 2002) masks from template space (Vemuri et al., 2008) to the softmean space. Grey matter ROIs that were defined on the softmean image were then applied to the annualized log Jacobian image. Based on our previous studies on PPAOS and agrammatic aphasia (Josephs et al., 2014; Josephs et al., 2013; Josephs et al., 2012; Whitwell, Duffy, Strand, Xia, et al., 2013), we assessed the following regions-of-interest: precentral cortex, paracentral cortex, supplementary motor area, Rolandic operculum, superior frontal gyrus, middle frontal gyrus, pars opercularis, pars triangularis, thalamus, caudate, putamen and pallidum.
Baseline assessment of grey matter volume at the voxel-level was assessed using voxel-based morphometry in SPM5 (Ashburner & Friston, 2000). All baseline MRI were normalized and segmented using a customized template and unified segmentation (Ashburner & Friston, 2005) and the grey matter images were modulated and smoothed at 10mm full-width-at-half-maximum. Two-sided t-tests were used to compare groups. Results were assessed corrected for multiple comparisons using the family wise error correction at p<0.05 and uncorrected at p<0.001.
2.5. Statistical analysis
Statistical analysis was performed using JMP 10.0.0. Statistical comparisons between groups were performed using Mann-Whitney U/Wilcoxon rank sum test for continuous variables and chi-squared tests for categorical variables. Annualized rates of change in the clinical measures were calculated as the number of points change between baseline and follow-up, divided by the follow-up interval in years. Regional rates of atrophy are expressed as annualized percentage decline from baseline.
3. RESULTS
Of the 20 PPAOS subjects, eight (40%) were determined to be aphasic at the follow-up visit (hereafter referred to as “progressors”). The remaining 12 subjects had not developed aphasia at the follow-up visit. Writing samples demonstrating the development of agrammatism in the progressors are shown in Table 1. The baseline characteristics of the PPAOS progressors and non-progressors are shown in Table 2. There were no differences in demographic features across the progressors and non-progressors, although the progressors were younger at onset. There was also no difference across groups in measures of cognition, functional ability, executive function, memory, visuoperceptual function, behavior, limb apraxia and Parkinsonism. Both groups showed a comparable degree of apraxia of speech at baseline, with median (inter-quartile range) apraxia of speech severity scores of 1 (1, 1.1) in the non-progressors and 1.5 (1, 2.3) in the progressors (p=0.38). While no subject showed any agrammatism in writing or speech at baseline, and all had normal scores on the WAB-AQ, the progressors did show a slightly lower WAB-fluency score at baseline. The apraxia of speech type differed significantly between groups at baseline, with apraxia of speech Type 1 only observed in the progressors. Annualized rates of change in the clinical scores are shown in Table 3. The progressors showed higher rates of change compared to the non-progressors in Token Test, WAB-AQ and WAB-fluency. Importantly, no difference in rate of change in apraxia of speech severity was observed between the progressors (0.5 (0.4, 0.6)) and non-progressors (0.5 (0.4, 0.7), p=0.97). There was no difference in the proportion of subjects with dysarthria between the non-progressors and progressors at baseline (two non-progressors (16%), one progressor (12.5%), p=0.80) or follow-up (six non-progressors (50%), two progressors (25%), p=0.26).
Table 1.
Writing samples from baseline and follow-up visits for the eight PPAOS progressors
| Patient | Baseline | Follow-up |
|---|---|---|
| 1 | "It's a weekend and the family is having a picnic by a lake." |
"Mom, dad, son are the dauter to going the picnic." |
| 2 | "There's a family at a picnic at the beach." |
"There is a lot activity on the beach and the lake." |
| 3 | "There is a man fishing off the dock and a young girl is making a sand castle." |
"The driveway of the house is car on it." |
| 4 | "The family was having a great day by the water." |
"The family went to lake weekend." |
| 5 | "The couple is having a picnic by the lake." |
"The girl is constructing sand castle while the couple is drinking wine." |
| 6 | "The little girl is on the beach playing in the sand and she has a pail & shovel beside her." |
"There a tree by house, & a car out front." |
| 7 | "Sammy is flying a kite while running. Sally is building a castle with a shovel and pail." |
"The mother was pouring wine and her husband was reading good book." |
| 8 | "Behind them is a house with a car in the driveway." |
"[Name] born Holland." (only writing > single word attempts) |
Table 2.
Subject demographics and baseline clinical data
| PPAOS non- progressors |
PPAOS progressors | Z/ChiSq‡ | P value | |
|---|---|---|---|---|
| N | 12 | 8 | ||
| No. female (%) | 5 (42%) | 4 (50%) | 0.1 | 0.71 |
| Education | 16 (15–18) | 15 (12–18) | −0.63 | 0.53 |
| Handedness (R/L/A) | 10 (83%)/ 2 (17%) | 5 (63%)/ 2 (25%)/ 1 (12%) | 2.0 | 0.38 |
| Age onset, years | 70 (66–73) | 61 (57–67) | −2.0 | 0.04 |
| Illness duration, years | 3 (2–4) | 4 (3–7) | 1.6 | 0.11 |
| Age baseline MRI, years | 75 (70,77) | 67 (62,70) | −1.6 | 0.11 |
| MRI interval, years | 2.1 (1.5 to 2.5) | 2.5 (2.2–2.7) | 1.0 | 0.31 |
| Time from baseline MRI to baseline clinical assessment, days |
0 (0–1) | 1 (1–1) | 2.0 | 0.05 |
| Time from follow-up MRI to follow-up clinical assessment, days |
1 (0–1) | 0 (0–1) | −1.0 | 0.34 |
| MMSE (/30) | 30 (29,30) | 29.5 (28.8,30) | −1.0 | 0.31 |
| CDR-SB (/18; 0=best) | 0 (0–0) | 0 (0–0.1) | −1.4 | 0.15 |
| FAB (/18) | 17 (16,17) | 16 (16,18) | −0.5 | 0.60 |
| Limb apraxia (/60) | 58 (57,59) | 58.5 (57.8,60) | 0.8 | 0.43 |
| UPDRS III (/132; 0=best) | 12.5 (5.8,16.8) | 6 (5,8) | −1.1 | 0.24 |
| NPI (/36; 0=best) | 1.5 (1,2.5) | 2 (0,5.3) | 0.2 | 0.88 |
| AVLT delayed recall* | 11.5 (11.0,14.0) | 11.5 (9.8,13.3) | −0.5 | 0.58 |
| DKEFS card sort* | 11 (10.3,13.3) | 12 (8.8,13.5) | 0.0 | 1.00 |
| VOSP letters (/20; 20=best) | 20 (20,20) | 20 (20,20) | −0.9 | 0.36 |
| Token Test V (/22) | 21 (20,22) | 20.5 (19,21.3) | −1.0 | 0.32 |
| WAB-AQ (/100) | 98.8 (96.4,100) | 96.3 (95.4,97.5) | −1.7 | 0.10 |
| WAB-fluency (/10) | 10 (9.8–10) | 9 (9–9.3) | −2.1 | 0.04 |
| WAB-info content (/10) | 10 (10,10) | 10 (10,10) | −1.1 | 0.26 |
| WAB-AV comp (/10) | 10 (10,10) | 10 (10,10) | −0.1 | 0.96 |
| BNT (/15) | 15 (13.8,15) | 15 (13.8,15) | −0.2 | 0.82 |
| Apraxia of speech type 1 (%)/ type 2 (%)† |
0 (0%)/ 12 (100%) | 5 (63%)/ 3 (37%) | 10 | 0.002 |
| AOS severity (/4) | 1.0 (1.0,1.1) | 1.5 (1.0,2.3) | 0.9 | 0.38 |
| MSD (/10) | 7 (7,8) | 7 (6,8) | −0.6 | 0.52 |
| Letter fluency (FAS, one min. each) | 22 (17.3,28.8) | 26 (18,32.3) | 0.5 | 0.59 |
| Animal fluency (in one min.) | 19 (15.5,20) | 18 (15,21) | 1.0 | 0.31 |
| Dysarthria severity (/4) | 0 (0,0.1) | 0 (0,0.1) | −0.1 | 0.96 |
Data shown as median (inter-quartile range); PPAOS = primary progressive apraxia of speech; MMSE = Mini-Mental State Examination; CDR-SB = Clinical Dementia Rating Sum of Boxes; FAB = Frontal Assessment Battery; UPDRS = Movement Disorders Society Sponsored revision of the Unified Parkinson’s Disease Rating Scale; NPI = Neuropsychiatric Inventory; AVLT = Auditory Verbal Learning Test; DKEFS = Delis-Kaplan Executive Function System; VOSP = Visual Object and Space Perception Battery; WAB = Western Aphasia Battery; WAB-AQ=WAB Aphasia Quotient; AV comp = auditory verbal comprehension; BNT = Boston Naming Test; AOS = apraxia of speech; MSD = Motor Speech Disorders severity scale
Scores are constructed to have a mean of 10 and standard deviation of 3 in cognitively healthy participants
At follow-up, one PPAOS progressor that was AOS type 2 at baseline was classified as AOS-NOS, and two PPAOS non-progressors that were AOS type 2 were classified as AOS-NOS. In all three patients, the designation of AOS-NOS at follow-up reflected the fact that they became too severe to judge whether Type 1 or Type 2 predominated.
For continuous variables, Z statistic reported from the large-sample approximation/tie correction of the Mann-Whitney U statistic/Wilcoxon rank sum statistic. For categorical variables, Chi Square test statistic reported.
Table 3.
Annualized rates of change in clinical scores
| PPAOS non- progressors |
PPAOS progressors |
Z* | P value | |
|---|---|---|---|---|
| MMSE | 0.0 (−0.5,0) | −0.2 (−0.9,0.1) | −0.1 | 0.94 |
| CDR-SB | 0 (0,1.0) | 0.1 (0,0.5) | 0.0 | 1.00 |
| FAB | −0.6 (−1.1,0) | −1.1 (−1.2,−0.9) | −1.2 | 0.24 |
| Limb apraxia | −1.5 (−2.8,0) | −2.4 (−3.6,−1.2) | −0.8 | 0.44 |
| UPDRS III | 6.9 (1.6,10.7) | 4.1 (1.2,7.5) | −0.5 | 0.59 |
| NPI | 0.2 (−0.7,0.5) | 0 (−0.6,0.7) | 0.2 | 0.79 |
| AVLT delayed recall | 0 (−0.8,0.4) | 0 (−0.3,0.6) | 0.2 | 0.85 |
| DKEFS card sort | 0.5 (−1.1,1.7) | 0 (−1.0,0.4) | −0.8 | 0.45 |
| VOSP letters | 0 (0,0) | 0 (0,0.3) | 1.4 | 0.16 |
| Token Test | 0 (−0.8,0) | −1.1 (−2.1,−0.8) | −2.1 | 0.03 |
| WAB-AQ | −1.2 (−1.4,−0.2) | −4.9 (−6.1,−2.5) | −2.1 | 0.03 |
| WAB-fluency | 0 (−0.4,0) | −1.7 (−2.1,−0.8) | −2.3 | 0.02 |
| WAB-info content | 0 (0,0) | 0 (−0.4,0) | −1.0 | 0.31 |
| WAB-AV comp | 0 (0,0) | 0 (−0.1,0) | −1.7 | 0.10 |
| BNT | 0 (−0.2,0) | −0.4 (−0.8,0) | −1.0 | 0.30 |
| MSD | −0.7 (−1.2,−0.4) | −0.8 (−1.2,−0.6) | −0.2 | 0.85 |
| AOS severity | 0.5 (0.4,0.7) | 0.5 (0.4,0.6) | −0.0 | 0.97 |
| Letter fluency | −2.1 (−4.0,−0.1) | −3.5 (−4.9,−0.3) | −0.3 | 0.77 |
| Animal fluency | −1.2 (−2.0,0.0) | −1.8 (−3.1,−1.0) | −1.0 | 0.30 |
| Dysarthria severity | 0.3 (0,0.4) | 0.1 (0,0.4) | −0.6 | 0.56 |
Data shown as median (inter-quartile range); All rates are expressed as points change per year (rate=repeat-baseline/interval). PPAOS = primary progressive apraxia of speech; MMSE = Mini-Mental State Examination; CDR-SB = Clinical Dementia Rating Sum of Boxes; FAB = Frontal Assessment Battery; UPDRS = Movement Disorders Society Sponsored revision of the Unified Parkinson’s Disease Rating Scale; NPI = Neuropsychiatric Inventory; AVLT = Auditory Verbal Learning Test; DKEFS = Delis-Kaplan Executive Function System; VOSP = Visual Object and Space Perception Battery; WAB = Western Aphasia Battery; WAB-AQ=WAB Aphasia Quotient; AV comp = auditory verbal comprehension; BNT = Boston Naming Test; AOS = apraxia of speech; MSD = Motor Speech Disorders severity scale
Z statistic reported from the large-sample approximation/tie correction of the Mann-Whitney U statistic/Wilcoxon rank sum statistic
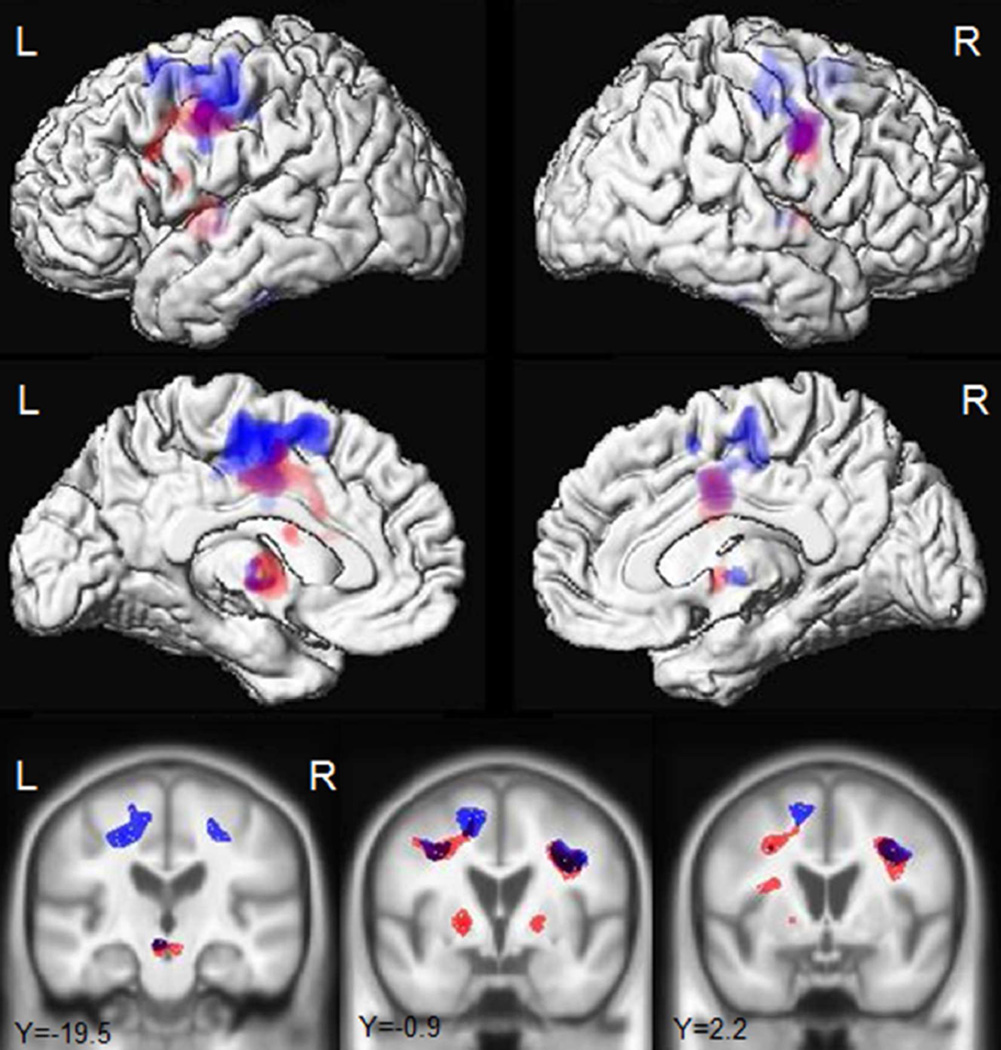
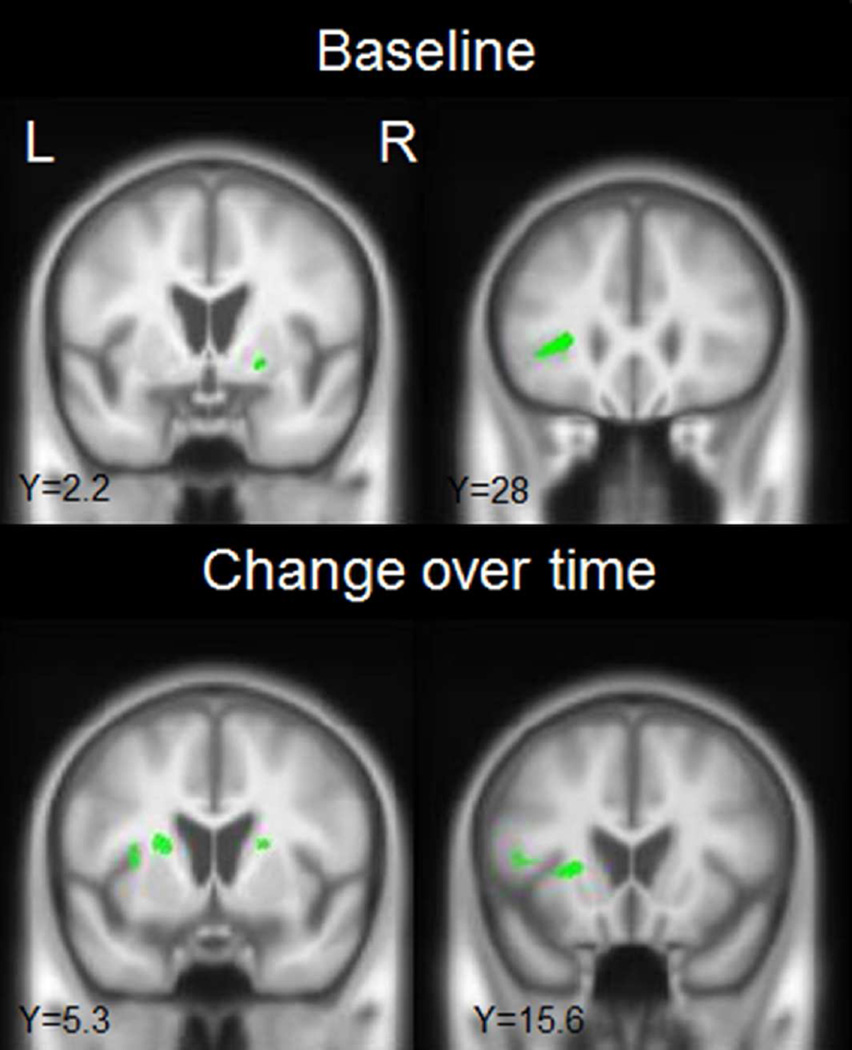
In the longitudinal neuroimaging analysis, the non-progressors showed striking grey matter atrophy bilaterally in premotor cortex, supplementary motor area, precentral cortex, paracentral lobule and frontal lobe (Fig. 1 and Table 4, p<0.05 corrected for multiple comparisons), particularly involving superior gyri (Fig. 1), compared to controls. These regions were also atrophic in the progressors compared to controls (Fig. 1 and Table 4, p<0.05 corrected for multiple comparisons). The two groups showed a similar degree of atrophy in precentral and paracentral cortices (Table 4), but rates of atrophy across the other frontal regions were generally greater in the progressors, particularly in the left hemisphere (Table 4). In addition to these frontal regions, both groups showed atrophy in the left thalamus, caudate nucleus, and putamen, as well as midbrain, compared to controls, although the progressors showed more prominent and bilateral involvement of the basal ganglia (Fig. 1 and Table 4). When the two groups were compared directly, the progressors showed significantly greater rates of atrophy in the left thalamus (p=0.03), left putamen (p=0.04), left pars triangularis (p=0.02), with a trend for greater rate in the left and right pars opercularis (p=0.05 and p=0.06), compared to the non-progressors (Table 4 and Fig. 2, uncorrected p<0.001). As a secondary analysis, a voxel-level regression analysis was performed correlating rates of atrophy to rates of change in WAB-fluency; the clinical measure that showed the most striking differences between groups. Change in WAB-fluency was associated with atrophy in the left inferior frontal lobe and basal ganglia (Supplemental Figure 1, uncorrected p<0.001).
Figure 1.
Longitudinal regions of grey matter atrophy in the progressors (red) and non-progressors (blue) compared to controls. Results are shown on lateral and medial three dimensional renderings of the brain and representative coronal slices. Results are shown corrected for multiple comparisons using the family wise error correction at p<0.05.
Table 4.
Annualized rates of grey matter atrophy
| Hem | Controls | PPAOS non- progressors |
PPAOS progressors | |
|---|---|---|---|---|
| Precentral | L | −0.16 (−0.60,0.24) | −3.20 (−3.48,−2.28)a | −3.20 (−3.96,−2.61)a |
| R | −0.19 (−0.65,0.37) | −2.66 (−3.36,−2.00)a | −2.44 (−3.36,−1.43)a | |
| Supplementary motor area | L | −0.24 (−0.72,0.28) | −2.99 (−4.87,−2.11)a | −3.87 (−4.50,−1.74)a |
| R | −0.39 (−0.85,0.08) | −2.80 (−3.38,−1.73)a | −2.30 (−4.50,−1.76)a | |
| Paracentral | L | −0.20 (−0.92,0.48) | −2.04 (−2.58,−0.99)a | −1.49 (−1.73,−0.88)a |
| R | −0.07 (−0.81,0.27) | −1.17 (−2.41,−0.65)a | −0.93 (−1.46,−0.54) | |
| Rolandic operculum | L | −0.26 (−0.86,0.26) | −0.72 (−1.74,0.10) | −1.53 (−1.65,−1.28)a |
| R | −0.22 (−0.55,0.35) | −0.94 (−2.09,−0.43)a | −1.34 (−1.75,−0.71)a | |
| Superior frontal | L | −0.10 (−0.91,0.33) | −1.33 (−1.85,−0.35)a | −2.01 (−2.74,−0.50)a |
| R | −0.20 (−0.73,0.28) | −1.19 (−2.03,−0.68)a | −1.31 (−2.36,−0.63) | |
| Middle frontal | L | −0.03 (−0.76,0.43) | −1.04 (−1.54,−0.37)a | −1.87 (−2.70,−0.71)a |
| R | −0.07 (−0.79,0.35) | −0.75 (−1.41,−0.42) | −1.07 (−1.49,−0.76)a | |
| Pars triangularis | L | −0.12 (−0.68,0.33) | −0.73 (−1.25,−0.21) | −1.96 (−2.53,−1.39)a,b |
| R | −0.22 (−0.88,0.36) | −0.87 (−1.44,−0.41) | −0.96(−1.65,−0.51) | |
| Pars opercularis | L | −0.16 (−0.75,0.28) | −1.07 (−1.96,−0.69)a | −2.62 (−3.20,−1.15)a |
| R | −0.11 (−0.34,0.37) | −1.03 (−1.41,−0.66)a | −1.77 (−2.21,−1.32)a | |
| Thalamus | L | −0.30 (−1.19,0.37) | −1.33 (−2.23,−0.79)a | −2.74 (−3.22,−2.30)a,b |
| R | −0.42 (−1.38,0.04) | −1.31 (−1.91,0.21) | −1.75 (−2.43,−0.53) | |
| Caudate | L | 0.02 (−0.37,0.32) | −1.04 (−1.32,−0.73)a | −1.27 (−1.77,−0.88)a |
| R | 0.27 (−0.31,0.91) | −0.91 (−1.65,−0.24)a | −1.33 (−1.55,−1.05)a | |
| Putamen | L | 0.05 (−0.48,0.65) | −0.62 (−1.06,0.06)a | −1.78 (−2.75,−1.23)a,b |
| R | −0.13 (−0.42,0.55) | −0.55 (−1.11,0.04) | −1.80 (−3.49,−0.79)a | |
| Pallidum | L | 0.45 (−0.11,1.86) | 1.42 (−1.46,2.60) | −1.23 (−2.15,−0.77)a |
| R | 1.55 (0.01,3.28) | 0.72 (−0.09,2.22) | −0.02 (−1.55,0.34)a |
Data shown as median (inter-quartile range); Regional rates of atrophy are expressed as annualized percentage decline from baseline.
Significantly different from controls;
Significantly different from non-progressors
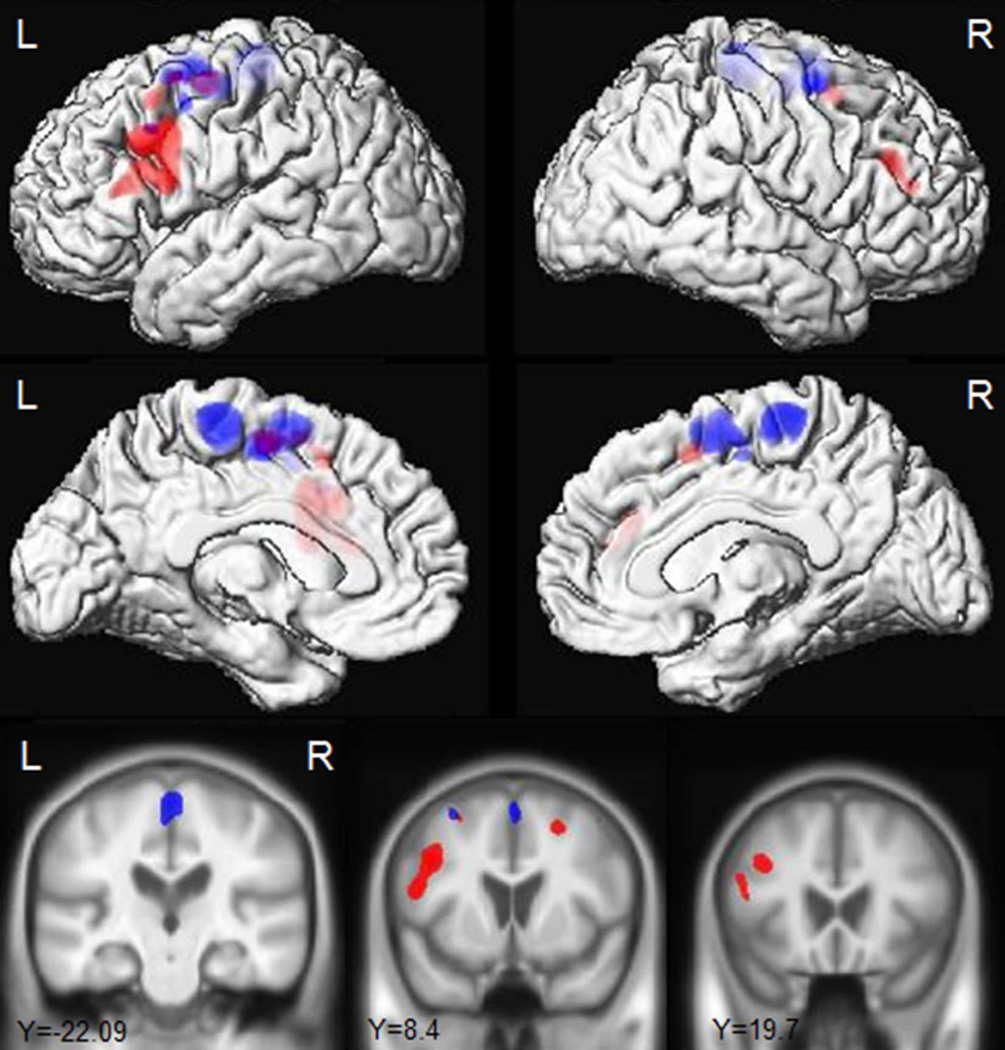
Figure 2.
Regions that showed greater grey matter atrophy in the progressors compared to the non-progressors at baseline and over time. Results are shown uncorrected for multiple comparisons at p<0.001.
The baseline findings did not survive correction for multiple comparisons and so are reported at an uncorrected threshold of p<0.001. The non-progressors showed grey matter loss bilaterally in the supplementary motor area, paracentral lobule and lateral superior premotor cortex compared to controls (Fig. 3). In contrast, the progressors showed grey matter loss predominantly in the left hemisphere, involving supplementary motor area, lateral superior, middle and inferior premotor cortex and putamen compared to controls (Fig. 3). The progressors showed greater grey matter loss in the left inferior triangularis and bilateral putamen compared to the non-progressors (Fig. 2). No regions showed greater grey matter loss in the non-progressors compared to the progressors.
Figure 3.
Regions of grey matter loss at baseline in the progressors (red) and non-progressors (blue) compared to controls. Results are shown on lateral and medial three dimensional renderings of the brain and representative coronal slices. Results are shown uncorrected for multiple comparisons at p<0.001.
4. DISCUSSION
This study provided us with the unique opportunity to assess the development of agrammatic aphasia in a cohort of subjects with PPAOS. Almost half of the cohort of 20 subjects developed aphasia over an interval of approximately two years, with this change associated with atrophy of Broca’s area, as well as the thalamus and basal ganglia.
The PPAOS subjects that developed agrammatic aphasia at the follow-up visit showed atrophy in a number of brain regions over the same time interval, with changes predominantly located in the left hemisphere and involving inferior, middle and superior frontal gyri, particularly in premotor regions, supplementary motor area, as well as the thalamus, basal ganglia and midbrain. Because these subjects also showed worsening over time in many other clinical features, such as apraxia of speech, Parkinsonism, and limb apraxia, the anatomic correlate of the agrammatic aphasia is difficult to disentangle. Importantly, however, this group of subjects was compared to a group of PPAOS subjects that showed comparable degrees of progression on apraxia of speech, Parkinsonism, and limb apraxia, but, critically, did not develop agrammatic aphasia. The findings from this comparison suggest that the left pars triangularis, thalamus and putamen are particularly associated with the development of agrammatism. There was also a trend for the left and right pars opercularis to show a faster rate of atrophy in the progressors compared to the non-progressors. These results were supported by the fact that rates of atrophy in the inferior frontal lobe and basal ganglia were also correlated with rate of change in WAB-fluency. Our findings concur with our previous study that found correlations between agrammatic aphasia and atrophy of the pars triangularis and opercularis (Whitwell, Duffy, Strand, Xia, et al., 2013); however, the involvement of the thalamus and basal ganglia suggests that Broca’s area may not be the only region associated with the development of agrammatic aphasia. Both the basal ganglia and thalamus have previously been implicated in agrammatic aphasia (Fridriksson, Bonilha, & Rorden, 2007; Marie, 1906; Wallesch et al., 1983), and, in fact, it has been suggested that Broca’s area basal ganglia thalamocortical circuitry may be important in language processing (Brunner, Kornhuber, Seemuller, Suger, & Wallesch, 1982; Ullman, 2001). It has been theorized that a frontal-striatal network may play a role in providing the executive resources required to comprehend complex syntax (Grossman, 1999) or in controlling rule-governed behavior, including the application of grammatical rules to combine morphemes into complex words (Ullman, 2001). Thalamic lesions can result in the presence of semantic paraphasias, although abnormalities in language comprehension and agrammatic non-fluent aphasia’s have also been reported (Crosson, 2013; Wallesch et al., 1983). Indeed, recent studies have demonstrated structural connectivity between Broca’s area and the thalamus and basal ganglia (Bohsali et al., 2015; Ford et al., 2013), with projections observed from both the pars opercularis and pars triangularis into the anterior putamen and the ventral anterior nucleus and pulvinar of the thalamus (Bohsali et al., 2015; Ford et al., 2013). Our findings support a role for this network in agrammatism.
There was evidence that this network of regions related to the development of agrammatism was already atrophic in the progressors at baseline compared to the non-progressors, despite the fact that agrammatism was not present clinically. These changes therefore appear to precede the development of agrammatism and represent a possible preclinical marker of the development of future agrammatism. These atrophic changes may have been present up to 2.5 years before the onset of agrammatism, although the exact preclinical interval will be best captured by future studies that follow PPAOS non-progressors for many years before some develop aphasia.
The speech and language data suggests the absence of agrammatism at baseline in the PPAOS subjects, with significant declines over time in the WAB and Token Test illustrating the development of agrammatism in the progressors. The WAB-fluency subtest which showed strikingly faster rates in the progressors is a construct in the WAB that includes grammatical abnormalities. The Token Test provides a measure of relatively complex sentence comprehension that includes a requirement for processing of grammatic and syntactic relationships (e.g. after picking up the green square, touch the white circle; touch-with the blue circle-the red square). The progressors did show slightly worse performance on the WAB-fluency variable at baseline, raising the possibility that some of them may have had subtle problems. Importantly, however, all still performed well; none received a fluency score that indicated the presence of agrammatic or telegraphic utterances (all subjects scored 9 or 10/10), and all scored within the normal range on the WAB-AQ. It is possible that slightly poor performance on the WAB fluency task could be an early indicator of the future development of agrammatism, although this might be difficult to detect in individual patients since performance was still within the normal range.
Interestingly, we observed a striking difference between the progressors and non-progressors in apraxia of speech type. Over 60% of the progressors were apraxia of speech type 1, meaning that they presented predominantly with distorted sound substitutions or additions. In contrast, none of the non-progressors had apraxia of speech type 1; all of them instead presented with a speech pattern predominated by syllable segmentation within multisyllabic words or across words, i.e. AOS type 2 (Josephs et al., 2013). The presence of apraxia of speech type 1 in PPAOS may, therefore, suggest that the subject is likely to develop agrammatic aphasia, while a subject with apraxia of speech type 2 would be less likely to develop agrammatic aphasia. This finding supports our previous study in which we found an association between type 1 and agrammatic aphasia. In the previous study, we found that apraxia of speech type 1 was more likely to occur in subjects that have dominant agrammatic aphasia rather than in those with a dominant apraxia of speech. It also supports the description of apraxia of speech by others when co-existent with aphasia (Ogar, Dronkers, Brambati, Miller, & Gorno-Tempini, 2007). These findings, together, highlight the importance of assessing the specific characteristics of the apraxia of speech. While this judgment is best made by neurologists and speech-language experts, it is possible that acoustic analysis of speech data may be sensitive to apraxia of speech type and may prove to be a useful diagnostic tool (J. R. Duffy et al., 2015). The PPAOS progressors were also younger at onset compared to the non-progressors, suggesting that young age in the context of PPAOS could prove to be a useful indicator of which subjects may develop agrammatism. A caveat, however, with both the age and apraxia of speech type findings is that we do not know whether any of the non-progressors will develop agrammatism in the future. Further follow-up will, therefore, be needed to determine the relationship between these clinical features and agrammatism.
The longitudinal assessment of subjects with PPAOS in this study has provided us with significant insight into the neurobiology underlying agrammatic aphasia. While the number of subjects in our study was relatively small, this is the largest cohort of PPAOS subjects to date that have been followed longitudinally. The unique nature of our cohort allowed us to assess the onset of agrammatism for the first time. It has also provided evidence that demographic and clinical features may differ between PPAOS progressors and non-progressors. Hence, if a PPAOS subject presents at a young age (~60 years) with apraxia of speech type 1, one could predict likely progression to agrammatic aphasia within the next couple of years. Understanding how the disease is likely to progress will be critically important to allow patients and their families to plan for the future.
Supplementary Material
Acknowledgments
We would like to thank Sarah Boland for study scheduling and Dr.’s Bradley F. Boeve, David S. Knopman, Jon Graff-Radford, and David T. Jones for seeing some of the normal control participants.
FUNDING
This work was supported by the National Institutes of Health [RO1-DC012519, RO1-DC010367 and U01-AG006786]. The funding source had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amici S, Ogar J, Brambati SM, Miller BL, Neuhaus J, Dronkers NL, Gorno-Tempini ML. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cognitive and behavioral neurology. 2007;20(4):203–211. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Bohsali AA, Triplett W, Sudhyadhom A, Gullett JM, McGregor K, FitzGerald DB, Crosson B. Broca's area - thalamic connectivity. Brain Lang. 2015;141:80–88. doi: 10.1016/j.bandl.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P. Sur le siege de la facultle due language articule. Bull Soc Anthropol. 1865;6:337–393. [Google Scholar]
- Brunner RJ, Kornhuber HH, Seemuller E, Suger G, Wallesch CW. Basal ganglia participation in language pathology. Brain Lang. 1982;16(2):281–299. doi: 10.1016/0093-934x(82)90087-6. [DOI] [PubMed] [Google Scholar]
- Crosson B. Thalamic mechanisms in language: a reconsideration based on recent findings and concepts. Brain Lang. 2013;126(1):73–88. doi: 10.1016/j.bandl.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p–linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (DKEFS): Examiner's manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca's historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130(Pt 5):1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: substrates, differetial diagnois, and management. 3rd. St Louis, MI: Mosby; 2013. [Google Scholar]
- Duffy JR, Strand EA, Clark H, Machulda M, Whitwell JL, Josephs KA. Primary progressive apraxia of speech: clinical features and acoustic and neurologic correlates. Am J Speech Lang Pathol. 2015;24(2):88–100. doi: 10.1044/2015_AJSLP-14-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan EP, Baker MC, Perkerson RB, Duffy JR, Strand EA, Whitwell JL, Josephs KA. Dominant frontotemporal dementia mutations in 140 cases of primary progressive aphasia and speech apraxia. Dement Geriatr Cogn Disord. 2015;39(5–6):281–286. doi: 10.1159/000375299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ford AA, Triplett W, Sudhyadhom A, Gullett J, McGregor K, Fitzgerald DB, Crosson B. Broca's area and its striatal and thalamic connections: a diffusion-MRI tractography study. Frontiers in neuroanatomy. 2013;7:8. doi: 10.3389/fnana.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Ridgway GR, Schott JM. Algorithms, atrophy and Alzheimer's disease: cautionary tales for clinical trials. Neuroimage. 2011;57(1):15–18. doi: 10.1016/j.neuroimage.2011.01.077. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Bonilha L, Rorden C. Severe Broca's aphasia without Broca's area damage. Behav Neurol. 2007;18(4):237–238. doi: 10.1155/2007/785280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Fillmore P, Guo D, Rorden C. Chronic Broca's Aphasia Is Caused by Damage to Broca's and Wernicke's Areas. Cereb Cortex. 2015;25(12):4689–4696. doi: 10.1093/cercor/bhu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170(3961):940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Duffy JR, Strand EA, Josephs KA. Parkinsonian motor features distinguish the agrammatic from logopenic variant of primary progressive aphasia. Parkinsonism & related disorders. 2012;18(7):890–892. doi: 10.1016/j.parkreldis.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. Sentence processing in Parkinson's disease. Brain and cognition. 1999;40(2):387–413. doi: 10.1006/brcg.1999.1087. [DOI] [PubMed] [Google Scholar]
- Grossman M, Powers J, Ash S, McMillan C, Burkholder L, Irwin D, Trojanowski JQ. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang. 2013;127(2):106–120. doi: 10.1016/j.bandl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Heutink P. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec J, Smith GE, Tangalos EG, Petersen RC, Kokmen E. Mayo's Older American Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56–97. The Clincial Neuropsychologist. 1992;6(supplement):1–104. [Google Scholar]
- Jack CR, Jr, Wiste HJ, Knopman DS, Vemuri P, Mielke MM, Weigand SD, Petersen RC. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82(18):1605–1612. doi: 10.1212/WNL.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodzio K, Gasecki D, Drumm DA, Lass P, Nyka W. Neuroanatomical correlates of the post-stroke aphasias studied with cerebral blood flow SPECT scanning. Med Sci Monit. 2003;9(3):MT32-41. [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, Whitwell JL. The evolution of primary progressive apraxia of speech. Brain. 2014;137(Pt 10):2783–2795. doi: 10.1093/brain/awu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, Whitwell JL. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81(4):337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, Whitwell JL. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery (Revised) San Antonio, Tx: PsychCorp.; 2007. [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(Pt 9):1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Archives of clinical neuropsychology. 1999;14(6):481–487. [PubMed] [Google Scholar]
- Lewis EB, Fox NC. Correction of differential intensity inhomogeneity in longitudinal MR images. Neuroimage. 2004;23(1):75–83. doi: 10.1016/j.neuroimage.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Marie P. The third frontal convolution plays no special role in the function of language. Sem Med. 1906;26:241–247. [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: pathologic and clinical. Neurology. 1978;28(4):311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer disease and associated disorders. 2007;21(4):S23–S30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, Fox NC. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72(18):1562–1569. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Mack JE. Grammatical Impairments in PPA. Aphasiology. 2014;28(8–9):1018–1037. doi: 10.1080/02687038.2014.912744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: the declarative/procedural model. Nat Rev Neurosci. 2001;2(10):717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Whitwell JL, Kantarci K, Josephs KA, Parisi JE, Shiung MS, Jack CR., Jr Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage. 2008;42(2):559–567. doi: 10.1016/j.neuroimage.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallesch CW, Kornhuber HH, Brunner RJ, Kunz T, Hollerbach B, Suger G. Lesions of the basal ganglia, thalamus, and deep white matter: differential effects on language functions. Brain Lang. 1983;20(2):286–304. doi: 10.1016/0093-934x(83)90046-9. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. The visual object and space perception battery. Bury St Edmonds, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, Josephs KA. Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. Eur J Neurol. 2013;20(4):629–637. doi: 10.1111/ene.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, Josephs KA. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013;125(3):245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZH, Zhao XQ, Wang CX, Chen HY, Zhang YM. Neuroanatomic correlation of the post-stroke aphasias studied with imaging. Neurol Res. 2008;30(4):356–360. doi: 10.1179/174313208X300332. [DOI] [PubMed] [Google Scholar]
- Yorkston K, Strand EA, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. J Medical Speech-Language Pathology. 1993;1(1):35–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.