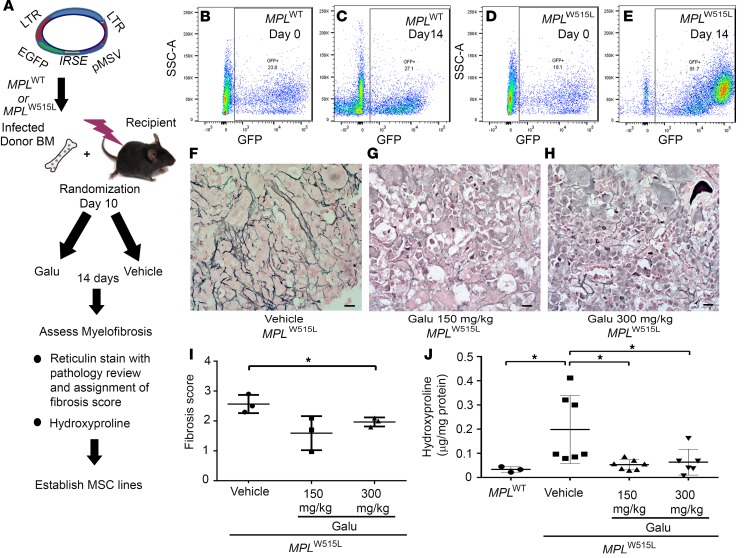
Figure 4. Galunisertib in vivo treatment significantly improves myelofibrosis of MPLW515L mice.
(A) Diagram showing the procedures of the experiments. Bones isolated from MPLW515L mice were randomized on day 10 to receive 1 of 2 doses of galunisertib (Galu) or vehicle control. Then, the mice were treated by oral gavage once daily for 14 days. (B–E) Representative flow cytometry staining of GFP+ retrovirally transduced bone marrow cells from MPLWT (B and C) and MPLW515L (D and E) on day 0 (representative mouse randomly selected and sacrificed) and on day 14 after treatment when all mice were sacrificed and further examined for pathological changes and for hydroxyproline quantification. Reticulin staining was then performed to evaluate fibrosis. Representative images of the reticulin stains are shown after treatment with vehicle (F), 150 mg/kg galunisertib (G), and 300 mg/kg galunisertib (H) to assess myelofibrosis in MPLW515L mice. Scale bars: 10 μm. (I) Fibrosis was scored according to European consensus on grading myelofibrosis (n = 3). *P < 0.05 by ANOVA, followed by Dunnett’s multiple comparison test. (J) Hydroxyproline amount was determined in the bones to quantify myelofibrosis in MPLWT-transduced mouse bone marrow, vehicle (n = 7), 150 mg/kg galunisertib (n = 7), and 300 mg/kg galunisertib (n = 6) groups. *P < 0.05 by ANOVA, followed by Dunnett’s multiple comparison test.