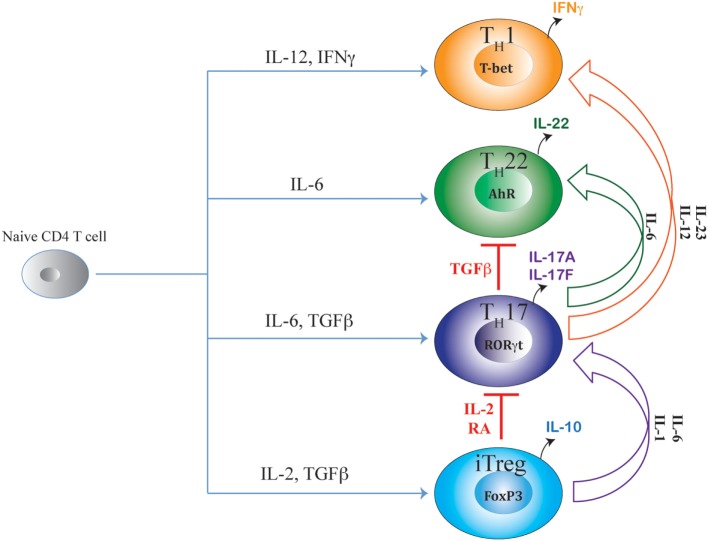
Figure 1.
Shared axes of Th17 differentiation. Developmental axis of Th17 subset substantially overlaps with developmental axes of iTreg, Th22, and Th1 subsets of T helper cells. While the origin of Th17 differentiation is intrinsically linked with iTreg cells due to their common requirement of TGFβ signaling, Th17 differentiation is also linked with Th22 subset due to the shared requirement of IL-6 signaling. Although proximal signaling events guiding Th17 differentiation are distinct from the Th1 subset, late developmental axis of Th17 is overlapping with Th1 cells as chronic TCR stimulation or action of IL-23 or IL-12 readily converts mature Th17 cells to IFNγ-producing “Th1-like” cells. Accordingly, along the entire developmental axis of Th17 and its related subsets, intermediate phenotypes co-producing FoxP3/IL-17, IL-17/IFNγ, and IL-17/IL-22 are found in vivo that can perform beneficial or pathogenic functions depending on the nature of the disease. While IL-2 and retinoic acid (RA) are negative regulators of iTreg–Th17 axis that oppose Th17 differentiation while promoting iTreg differentiation, TGFβ negatively regulates the Th17–Th22 axis as well as the Th17–Th1 axis by suppressing Th22 and Th1 cellular differentiations while facilitating iTreg and Th17 differentiations.