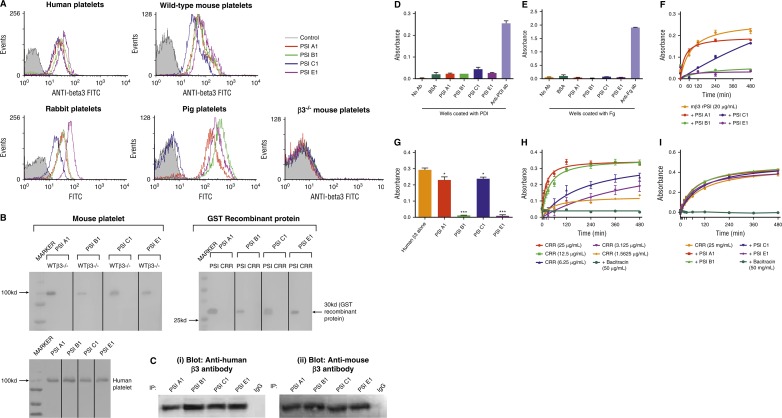
Figure 3.
Anti-β3 PSI domain antibodies bind specifically to the PSI domain of β3 integrin. (A) Flow cytometry demonstrated that anti-β3 PSI mAbs (PSI A1, PSI B1, PSI C1, and PSI E1) bound to β3 on platelets: wild-type or β3−/− murine platelets, human, pig, or rabbit platelets were incubated with PBS (control) or anti-PSI mAbs followed by incubation with fluorescein isothiocyanate–conjugated anti-mouse IgG. The binding was tested using flow cytometry. (B) The mAbs (PSI A1, PSI B1, PSI C1, and PSI E1) recognize the linear epitope of β3-integrin PSI domain. The recombinant PSI domain or the lysates from wild-type or β3−/− platelet were loaded into a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis in reducing conditions. After electrophoresis and transfer, the polyvinylidene fluoride membrane was cut and each strip was incubated with a different anti-PSI mAb, as indicated in the figure, followed by alkaline phosphatase (AP) or HRP-conjugated anti-mouse IgG. The binding was detected by 5-bromo-4-chloro-3-indolyl phosphate-toluidine salt/nitro blue tetrazolium–purple liquid (for AP) or chemiluminescent substrate following manufacturers’ instructions. (C) Immunoprecipitation further demonstrated that anti-mouse β3 PSI domain mAbs specifically bound the β3-integrin PSI domain. The anti-PSI mAbs were used for the immunoprecipitation and the commercial anti-human (i) or anti-mouse β3 integrin (ii) antibodies were used for the western blot. NIT G, an anti-GPIb mAb, was used as a negative IgG control. 1 × 108 platelets were used. (D-E) Anti-PSI mAbs did not bind PDI (D) or fibrinogen (E). ELISA plate wells were coated with PDI (1 μg/mL) or fibrinogen (0.1 μg/mL) overnight. After blocking with bovine serum antigen (1 μg/mL) for 1 hour, anti-PSI mAbs (1 μg/mL), anti-PDI antibody (positive control), or anti-fibrinogen antibody (positive control) was added and incubated for 1.5 hours at 37°C. After addition of HRP-conjugated secondary antibody, substrate was added and absorbance was measured at 492 nm. (F) Anti-PSI mAbs significantly inhibited mouse β3-integrin recombinant PSI thiol-isomerase function (mean ± SEM; absorbance after 480 minutes from different groups was compared; PSI A1 and PSI C1 vs rPSI: P < .05; PSI B1 and PSI E1 vs rPSI: P < .01; n = 3 each). (G) Anti-PSI mAbs (5 μg/mL) inhibited purified native human β3-integrin (40 μg/mL) thiol-isomerase function (mean ± SEM; *P < .05, ***P < .001; n = 3). (H-I) Anti-PSI mAbs did not inhibit CRR PDI-like activity. CRR has endogenous PDI-like activity (H). CRR was incubated with or without anti-PSI mAbs (5 μg/mL) followed by adding rdRNase overnight. The PDI-like activity was measured using rdRNase assay.