Figure 8.
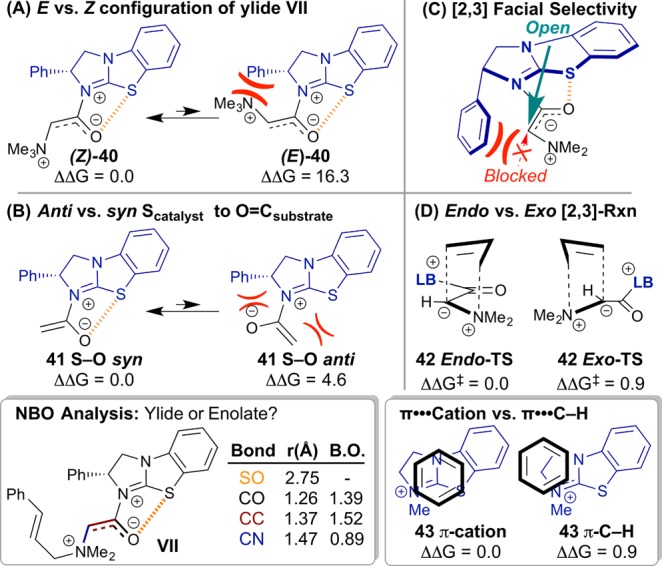
Computed model systems (all energies in kcal·mol–1 and distances in Å). (A) Preference for Z over E enolate. Enolate-like character indicated by bond orders (B. O.) estimated from the Wiberg bond indices (bottom left inset). (B) Effect of S···O interaction on acylated catalyst conformation. (C) With the acylated catalyst conformation held rigid (S···O), the BTM stereodirecting Ph sterically biases open enolate face. (D) Endo rearrangement is favored. In the major TS, this preference is reinforced by a π–cation interaction (bottom right inset).
