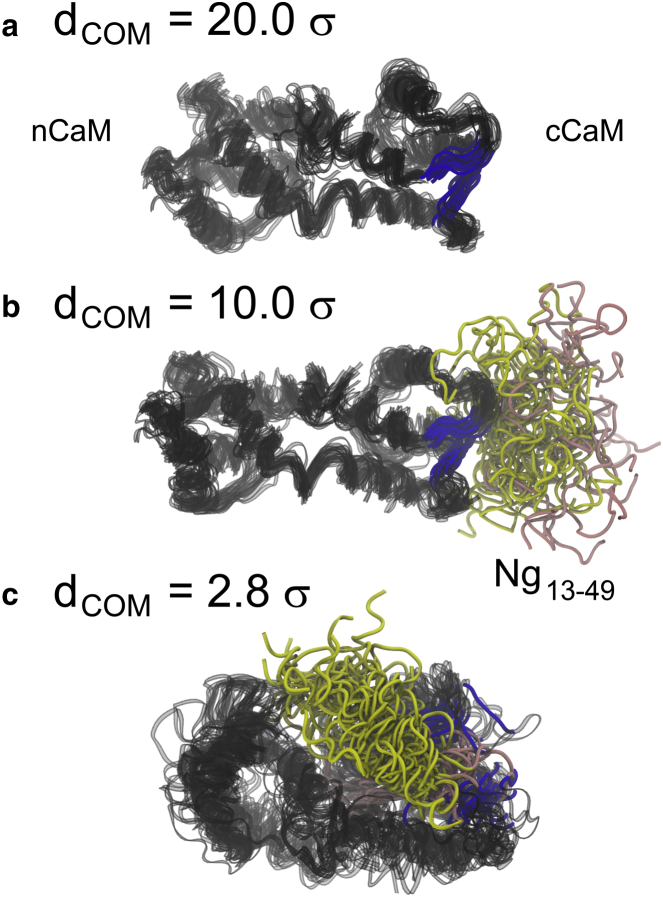
Figure 3.
Illustration of the structural changes in unbound, encounter, and bound ensemble of CaM-Ng13–49 complexes. Structures of CaM in (a) was taken from the unbound state when CaM and Ng13–49 are well separated at dCOM = 20.0 σ. σ = 3.8 Å. (b) Structures from the encounter of binding when apoCaM and Ng is separated at dCOM = 10.0 σ. (c) Structures from the bound state at dCOM = 2.8 σ. For visual guidance, we superposed 20 sets of structures in each panel. The CaM is colored in black; the residues (residues 99∼101 in Ca2+ binding loop III and residues 135∼137 in Ca2+ binding loop IV), which form EF-hand β-scaffold in cCaM are colored in blue; the acidic region and IQ motif of Ng13–49 are colored in pink and yellow, respectively. To see this figure in color, go online.