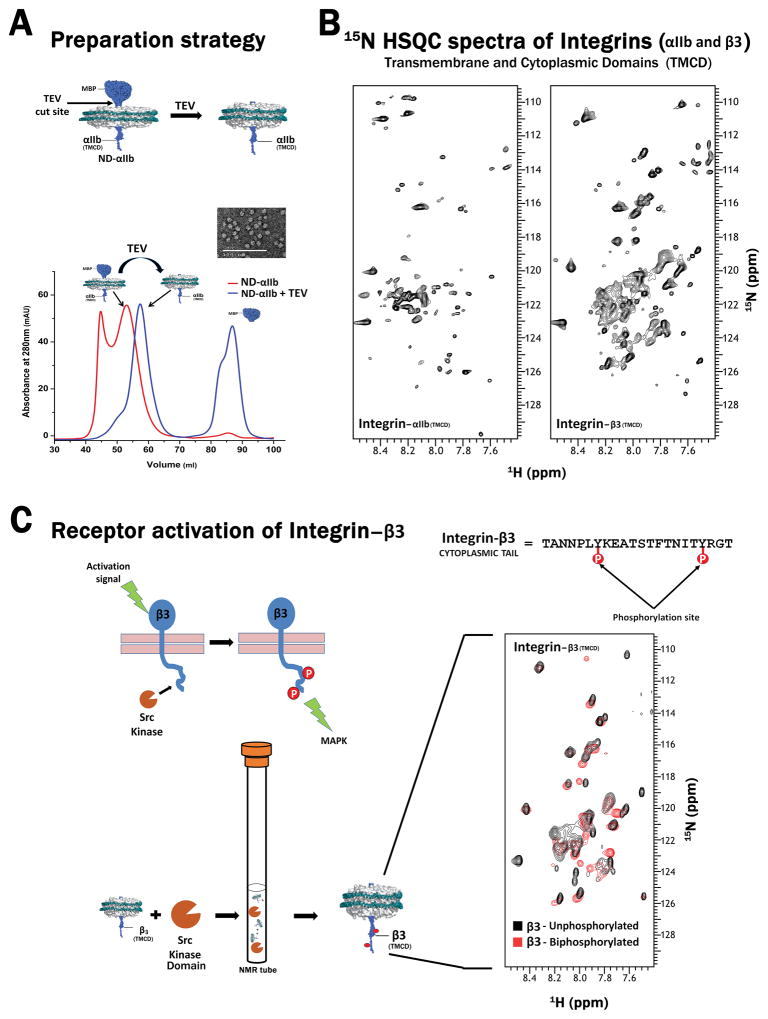
Figure 4.
(A) Overview of the procedure to obtain Integrin αIIb [trans-membrane and cytoplasmic domains (TMCD)] incorporated nanodiscs. TEV protease treatment of MBP- Integrin αIIb (TMCD) incorporated nanodiscs, cleaves the MBP–tag at the TEV cleavage site engineered between the tag and Integrin. The αIIb incorporated discs can then be purified from the rest of the cut products using SEC. Inset: Negatively stained TEM image of αIIb containing nanodiscs displaying a homogeneous population of discs. The exact same procedure can also be extended to obtain β3 (TMCD) incorporated nanodiscs. (B) 1H-15N- TROSY HSQC spectra of Integrin αIIb and β3 (TMCD) acquired on 800 MHz magnet (Agilent, USA). The sharp peaks correspond to the cytoplasmic tail region while the broader peaks correspond to the transmembrane region. (C) Applicability of nanodisc systems for studying signaling pathways. A schematic showing the activation events of Integrin β3 which is relayed intracellularly through the phosphorylation of its cytoplasmic tail by Src kinase (Top Panel). This event is mimicked (in vitro) in an NMR tube by mixing 15N labeled β3 incorporated discs with the kinase domain of Src kinase in the presence of ATP molecules. Phosphorylation is manifested through chemical shift perturbations (CSP) observed through the overlay of the 1H-15N- TROSY HSQC spectra obtained from the un-phosphorylated (black) and bi-phosphorylated (red) β3 (TMCD) collected on 600 MHz magnet (Agilent, USA). Also shown is the sequence of the β3 tail where the phosphorylation occurs at the two tyrosine residues.