Figure 3.
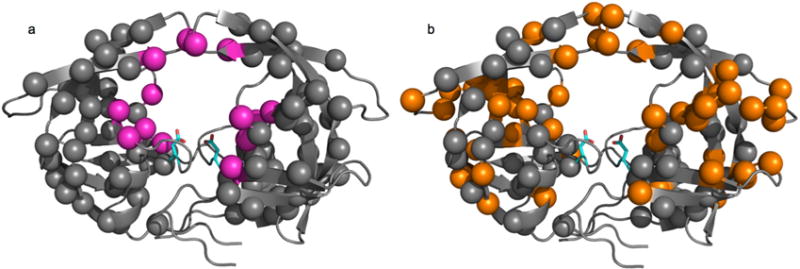
Location of drug resistance and conformational mutations in HIV-1 PR. Structural mapping of various mutations on the crystal structure of apo HIV-1 PR (1HHR). The catalytic Asp25 residue is depicted as cyan sticks on each enzyme protomer. All spheres represent a residue documented to undergo mutation in HIV-1 PR [97]. (a) Location of drug resistance-inducing mutations. Magenta spheres represent mutations that impair inhibitor binding through direct active-site contacts (primary mutations) and gray spheres represent distal indirect mutations (secondary mutations); (b) Location of mutations documented to affect the conformational sampling of HIV-1 PR, depicted as orange spheres. Gray spheres are other drug resistance-inducing mutations. All mutations were compiled from references listed in the text.
