Fig. 6. Paraestrol A docking into the pocket of the ancestral steroid receptor.
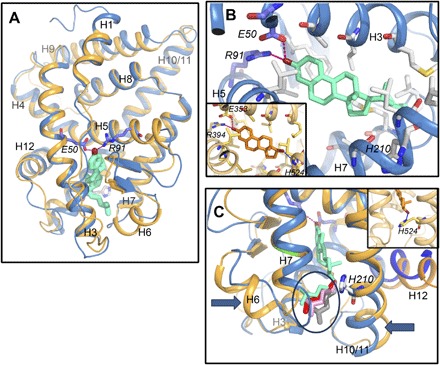
(A) Overall view of the homology model of AncSR LBD based on the crystal structure of ERα LBD in complex with 17β-estradiol (PDB code, 1ERE). Paraestrol A (green) is docked inside and binds to the receptor through the residues E50 and R91. Similar results are obtained using the crystal structure of the ancestral corticoid receptor in complex with desoxycorticosterone (PDB code, 2Q3Y) as a template (light orange trace). Both traces are from AncSR_D, with the traces from AncSR_AC being identical. (B) Detailed view on the binding pocket. Compared to the binding of 17β-estradiol (orange, inset), paraestrol A lacks the possibility of binding H524 from the receptor through its 17β-hydroxylated carbon. (C) Because of lack of binding to H210, the residue homologous to H524 in ERα (orange, inset), the aliphatic chain of paraestrol A wobbles inside the pocket. A few among many possible conformations are represented by different colors (encircled).
